Abstract
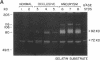
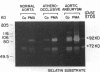
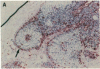
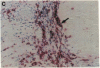
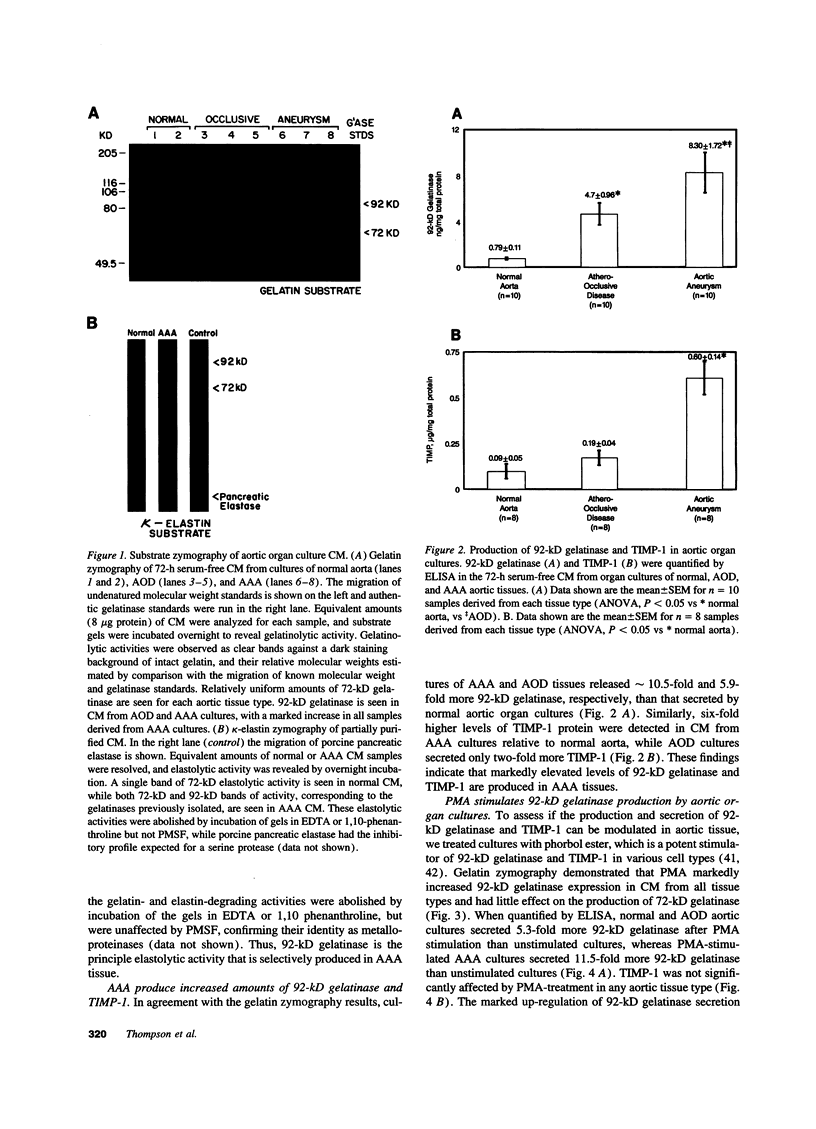
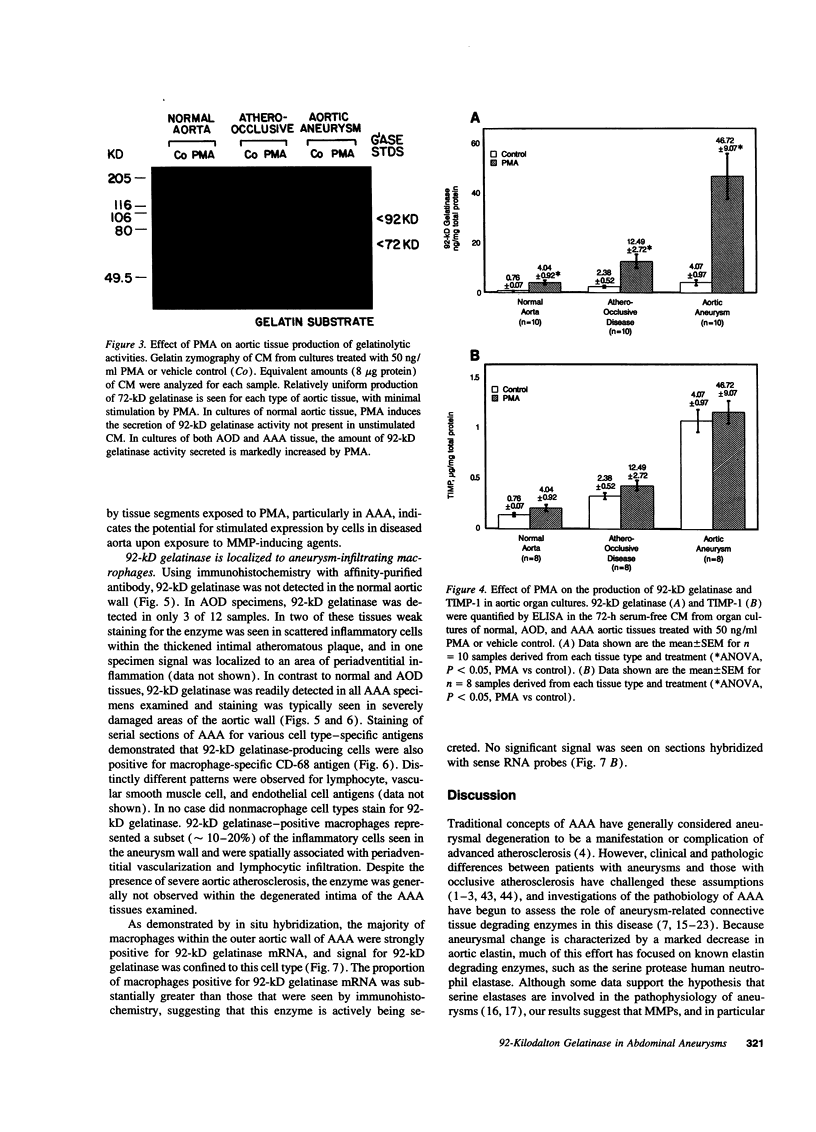
Abdominal aortic aneurysms (AAA) are characterized by disruption and degradation of the elastic media, yet the elastolytic proteinases involved and their cellular sources are undefined. We examined if 92-kD gelatinase, an elastolytic matrix metalloproteinase, participates in the pathobiology of AAA. Gelatin zymography of conditioned medium from normal, atheroocclusive disease (AOD), or AAA tissues in organ culture showed that all tissues produced 72-kD gelatinase. AOD and AAA cultures also secreted 92-kD gelatinase, but significantly more enzyme was released from AAA tissues. ELISA confirmed that AAA tissues released approximately 2-fold more 92-kD gelatinase than AOD tissue and approximately 10-fold more than normal aorta. Phorbol ester induced a 5.3-fold increase in 92-kD gelatinase secretion by normal aorta and AOD and an 11.5-fold increase by AAA. By immunohistochemistry, 92-kD gelatinase was not detected in normal aorta and was only occasionally seen within the neointimal lesions of AOD tissue. In all AAA specimens, however, 92-kD gelatinase was readily localized to numerous macrophages in the media and at the adventitial-medial junction. The expression of 92-kD gelatinase mRNA by aneurysm-infiltrating macrophages was confirmed by in situ hybridization. These results demonstrate that diseased aortic tissues secrete greater amounts of gelatinolytic activity than normal aorta primarily due to increased production of 92-kD gelatinase. In addition, the localization of 92-kD gelatinase to macrophages in the damaged wall of aneurysmal aortas suggests that chronic release of this elastolytic metalloproteinase contributes to extracellular matrix degradation in AAA.
Full text
PDF


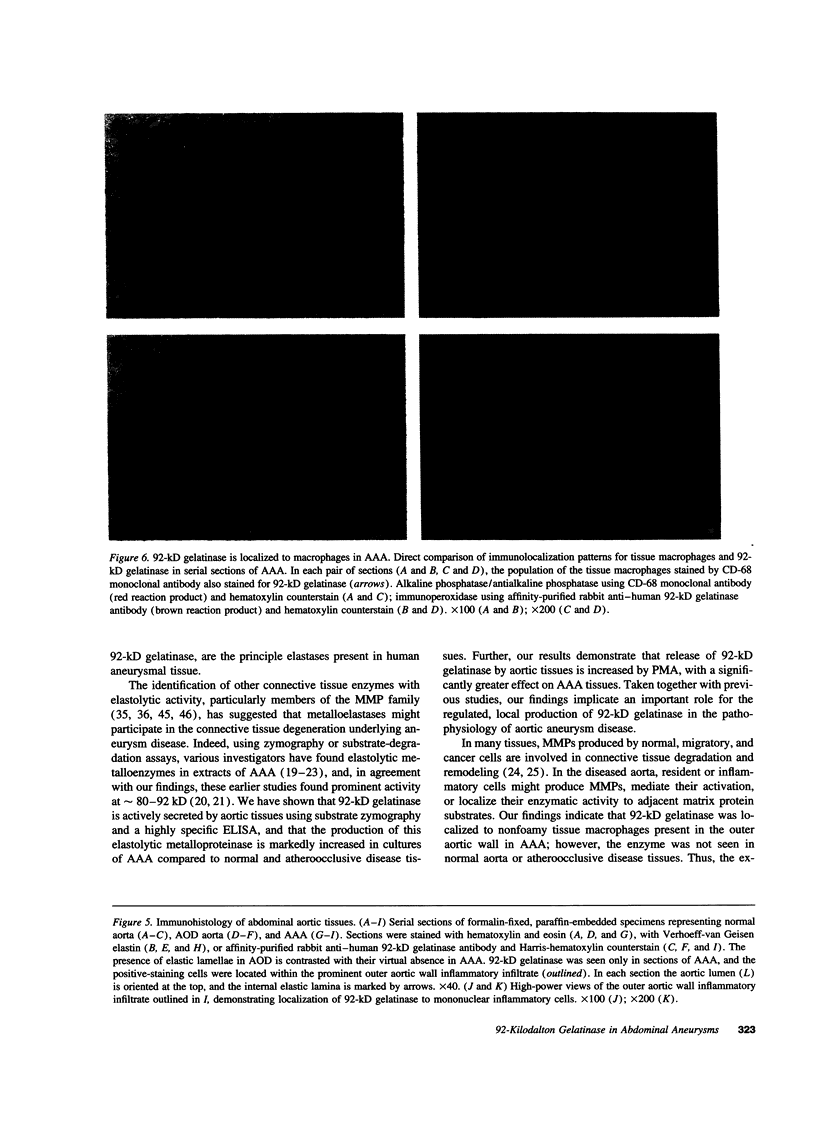



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angel P., Baumann I., Stein B., Delius H., Rahmsdorf H. J., Herrlich P. 12-O-tetradecanoyl-phorbol-13-acetate induction of the human collagenase gene is mediated by an inducible enhancer element located in the 5'-flanking region. Mol Cell Biol. 1987 Jun;7(6):2256–2266. doi: 10.1128/mcb.7.6.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anidjar S., Kieffer E. Pathogenesis of acquired aneurysms of the abdominal aorta. Ann Vasc Surg. 1992 May;6(3):298–305. doi: 10.1007/BF02000279. [DOI] [PubMed] [Google Scholar]
- Botney M. D., Kaiser L. R., Cooper J. D., Mecham R. P., Parghi D., Roby J., Parks W. C. Extracellular matrix protein gene expression in atherosclerotic hypertensive pulmonary arteries. Am J Pathol. 1992 Feb;140(2):357–364. [PMC free article] [PubMed] [Google Scholar]
- Brophy C. M., Reilly J. M., Smith G. J., Tilson M. D. The role of inflammation in nonspecific abdominal aortic aneurysm disease. Ann Vasc Surg. 1991 May;5(3):229–233. doi: 10.1007/BF02329378. [DOI] [PubMed] [Google Scholar]
- Campa J. S., Greenhalgh R. M., Powell J. T. Elastin degradation in abdominal aortic aneurysms. Atherosclerosis. 1987 May;65(1-2):13–21. doi: 10.1016/0021-9150(87)90003-7. [DOI] [PubMed] [Google Scholar]
- Campbell E. J., Cury J. D., Shapiro S. D., Goldberg G. I., Welgus H. G. Neutral proteinases of human mononuclear phagocytes. Cellular differentiation markedly alters cell phenotype for serine proteinases, metalloproteinases, and tissue inhibitor of metalloproteinases. J Immunol. 1991 Feb 15;146(4):1286–1293. [PubMed] [Google Scholar]
- Cohen J. R., Mandell C., Chang J. B., Wise L. Elastin metabolism of the infrarenal aorta. J Vasc Surg. 1988 Feb;7(2):210–214. doi: 10.1067/mva.1988.avs0070210. [DOI] [PubMed] [Google Scholar]
- Cohen J. R., Sarfati I., Danna D., Wise L. Smooth muscle cell elastase, atherosclerosis, and abdominal aortic aneurysms. Ann Surg. 1992 Sep;216(3):327–332. doi: 10.1097/00000658-199209000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst C. B. Abdominal aortic aneurysm. N Engl J Med. 1993 Apr 22;328(16):1167–1172. doi: 10.1056/NEJM199304223281607. [DOI] [PubMed] [Google Scholar]
- Goldberg G. I., Strongin A., Collier I. E., Genrich L. T., Marmer B. L. Interaction of 92-kDa type IV collagenase with the tissue inhibitor of metalloproteinases prevents dimerization, complex formation with interstitial collagenase, and activation of the proenzyme with stromelysin. J Biol Chem. 1992 Mar 5;267(7):4583–4591. [PubMed] [Google Scholar]
- Herron G. S., Unemori E., Wong M., Rapp J. H., Hibbs M. H., Stoney R. J. Connective tissue proteinases and inhibitors in abdominal aortic aneurysms. Involvement of the vasa vasorum in the pathogenesis of aortic aneurysms. Arterioscler Thromb. 1991 Nov-Dec;11(6):1667–1677. doi: 10.1161/01.atv.11.6.1667. [DOI] [PubMed] [Google Scholar]
- Hibbs M. S., Hasty K. A., Seyer J. M., Kang A. H., Mainardi C. L. Biochemical and immunological characterization of the secreted forms of human neutrophil gelatinase. J Biol Chem. 1985 Feb 25;260(4):2493–2500. [PubMed] [Google Scholar]
- Hollier L. H., Taylor L. M., Ochsner J. Recommended indications for operative treatment of abdominal aortic aneurysms. Report of a subcommittee of the Joint Council of the Society for Vascular Surgery and the North American Chapter of the International Society for Cardiovascular Surgery. J Vasc Surg. 1992 Jun;15(6):1046–1056. [PubMed] [Google Scholar]
- Koch A. E., Haines G. K., Rizzo R. J., Radosevich J. A., Pope R. M., Robinson P. G., Pearce W. H. Human abdominal aortic aneurysms. Immunophenotypic analysis suggesting an immune-mediated response. Am J Pathol. 1990 Nov;137(5):1199–1213. [PMC free article] [PubMed] [Google Scholar]
- Koch A. E., Kunkel S. L., Pearce W. H., Shah M. R., Parikh D., Evanoff H. L., Haines G. K., Burdick M. D., Strieter R. M. Enhanced production of the chemotactic cytokines interleukin-8 and monocyte chemoattractant protein-1 in human abdominal aortic aneurysms. Am J Pathol. 1993 May;142(5):1423–1431. [PMC free article] [PubMed] [Google Scholar]
- Lacraz S., Nicod L., Galve-de Rochemonteix B., Baumberger C., Dayer J. M., Welgus H. G. Suppression of metalloproteinase biosynthesis in human alveolar macrophages by interleukin-4. J Clin Invest. 1992 Aug;90(2):382–388. doi: 10.1172/JCI115872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefevre M., Rucker R. B. Aorta elastin turnover in normal and hypercholesterolemic Japanese quail. Biochim Biophys Acta. 1980 Jul 15;630(4):519–529. doi: 10.1016/0304-4165(80)90006-9. [DOI] [PubMed] [Google Scholar]
- Mackay A. R., Ballin M., Pelina M. D., Farina A. R., Nason A. M., Hartzler J. L., Thorgeirsson U. P. Effect of phorbol ester and cytokines on matrix metalloproteinase and tissue inhibitor of metalloproteinase expression in tumor and normal cell lines. Invasion Metastasis. 1992;12(3-4):168–184. [PubMed] [Google Scholar]
- Majumder P. P., St Jean P. L., Ferrell R. E., Webster M. W., Steed D. L. On the inheritance of abdominal aortic aneurysm. Am J Hum Genet. 1991 Jan;48(1):164–170. [PMC free article] [PubMed] [Google Scholar]
- Marshall B. C., Santana A., Xu Q. P., Petersen M. J., Campbell E. J., Hoidal J. R., Welgus H. G. Metalloproteinases and tissue inhibitor of metalloproteinases in mesothelial cells. Cellular differentiation influences expression. J Clin Invest. 1993 Apr;91(4):1792–1799. doi: 10.1172/JCI116390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matrisian L. M. Metalloproteinases and their inhibitors in matrix remodeling. Trends Genet. 1990 Apr;6(4):121–125. doi: 10.1016/0168-9525(90)90126-q. [DOI] [PubMed] [Google Scholar]
- McGee G. S., Baxter B. T., Shively V. P., Chisholm R., McCarthy W. J., Flinn W. R., Yao J. S., Pearce W. H. Aneurysm or occlusive disease--factors determining the clinical course of atherosclerosis of the infrarenal aorta. Surgery. 1991 Aug;110(2):370–376. [PubMed] [Google Scholar]
- Murphy G., Cockett M. I., Ward R. V., Docherty A. J. Matrix metalloproteinase degradation of elastin, type IV collagen and proteoglycan. A quantitative comparison of the activities of 95 kDa and 72 kDa gelatinases, stromelysins-1 and -2 and punctuated metalloproteinase (PUMP). Biochem J. 1991 Jul 1;277(Pt 1):277–279. doi: 10.1042/bj2770277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy G., Ward R., Gavrilovic J., Atkinson S. Physiological mechanisms for metalloproteinase activation. Matrix Suppl. 1992;1:224–230. [PubMed] [Google Scholar]
- Okada Y., Katsuda S., Okada Y., Nakanishi I. An elastinolytic enzyme detected in the culture medium of human arterial smooth muscle cells. Cell Biol Int. 1993 Sep;17(9):863–869. doi: 10.1006/cbir.1993.1149. [DOI] [PubMed] [Google Scholar]
- Pearce W. H., Sweis I., Yao J. S., McCarthy W. J., Koch A. E. Interleukin-1 beta and tumor necrosis factor-alpha release in normal and diseased human infrarenal aortas. J Vasc Surg. 1992 Nov;16(5):784–789. [PubMed] [Google Scholar]
- Powell J. T., Bashir A., Dawson S., Vine N., Henney A. M., Humphries S. E., Greenhalgh R. M. Genetic variation on chromosome 16 is associated with abdominal aortic aneurysm. Clin Sci (Lond) 1990 Jan;78(1):13–16. doi: 10.1042/cs0780013. [DOI] [PubMed] [Google Scholar]
- Powell J., Greenhalgh R. M. Cellular, enzymatic, and genetic factors in the pathogenesis of abdominal aortic aneurysms. J Vasc Surg. 1989 Feb;9(2):297–304. doi: 10.1067/mva.1989.vs0090297. [DOI] [PubMed] [Google Scholar]
- Reed D., Reed C., Stemmermann G., Hayashi T. Are aortic aneurysms caused by atherosclerosis? Circulation. 1992 Jan;85(1):205–211. doi: 10.1161/01.cir.85.1.205. [DOI] [PubMed] [Google Scholar]
- Reilly J. M., Brophy C. M., Tilson M. D. Characterization of an elastase from aneurysmal aorta which degrades intact aortic elastin. Ann Vasc Surg. 1992 Nov;6(6):499–502. doi: 10.1007/BF02000820. [DOI] [PubMed] [Google Scholar]
- Reilly J. M., Sicard G. A., Lucore C. L. Abnormal expression of plasminogen activators in aortic aneurysmal and occlusive disease. J Vasc Surg. 1994 May;19(5):865–872. doi: 10.1016/s0741-5214(94)70012-5. [DOI] [PubMed] [Google Scholar]
- Rizzo R. J., McCarthy W. J., Dixit S. N., Lilly M. P., Shively V. P., Flinn W. R., Yao J. S. Collagen types and matrix protein content in human abdominal aortic aneurysms. J Vasc Surg. 1989 Oct;10(4):365–373. doi: 10.1067/mva.1989.13151. [DOI] [PubMed] [Google Scholar]
- Saarialho-Kere U. K., Chang E. S., Welgus H. G., Parks W. C. Distinct localization of collagenase and tissue inhibitor of metalloproteinases expression in wound healing associated with ulcerative pyogenic granuloma. J Clin Invest. 1992 Nov;90(5):1952–1957. doi: 10.1172/JCI116073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saarialho-Kere U. K., Chang E. S., Welgus H. G., Parks W. C. Expression of interstitial collagenase, 92-kDa gelatinase, and tissue inhibitor of metalloproteinases-1 in granuloma annulare and necrobiosis lipoidica diabeticorum. J Invest Dermatol. 1993 Mar;100(3):335–342. doi: 10.1111/1523-1747.ep12470032. [DOI] [PubMed] [Google Scholar]
- Saarialho-Kere U. K., Kovacs S. O., Pentland A. P., Olerud J. E., Welgus H. G., Parks W. C. Cell-matrix interactions modulate interstitial collagenase expression by human keratinocytes actively involved in wound healing. J Clin Invest. 1993 Dec;92(6):2858–2866. doi: 10.1172/JCI116906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior R. M., Griffin G. L., Fliszar C. J., Shapiro S. D., Goldberg G. I., Welgus H. G. Human 92- and 72-kilodalton type IV collagenases are elastases. J Biol Chem. 1991 Apr 25;266(12):7870–7875. [PubMed] [Google Scholar]
- Senior R. M., Griffin G. L., Mecham R. P. Chemotactic activity of elastin-derived peptides. J Clin Invest. 1980 Oct;66(4):859–862. doi: 10.1172/JCI109926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior R. M., Griffin G. L., Mecham R. P., Wrenn D. S., Prasad K. U., Urry D. W. Val-Gly-Val-Ala-Pro-Gly, a repeating peptide in elastin, is chemotactic for fibroblasts and monocytes. J Cell Biol. 1984 Sep;99(3):870–874. doi: 10.1083/jcb.99.3.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro S. D., Endicott S. K., Province M. A., Pierce J. A., Campbell E. J. Marked longevity of human lung parenchymal elastic fibers deduced from prevalence of D-aspartate and nuclear weapons-related radiocarbon. J Clin Invest. 1991 May;87(5):1828–1834. doi: 10.1172/JCI115204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro S. D., Kobayashi D. K., Ley T. J. Cloning and characterization of a unique elastolytic metalloproteinase produced by human alveolar macrophages. J Biol Chem. 1993 Nov 15;268(32):23824–23829. [PubMed] [Google Scholar]
- Ståhle-Bäckdahl M., Inoue M., Guidice G. J., Parks W. C. 92-kD gelatinase is produced by eosinophils at the site of blister formation in bullous pemphigoid and cleaves the extracellular domain of recombinant 180-kD bullous pemphigoid autoantigen. J Clin Invest. 1994 May;93(5):2022–2030. doi: 10.1172/JCI117196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ståhle-Bäckdahl M., Parks W. C. 92-kd gelatinase is actively expressed by eosinophils and stored by neutrophils in squamous cell carcinoma. Am J Pathol. 1993 Apr;142(4):995–1000. [PMC free article] [PubMed] [Google Scholar]
- Tilson M. D., Stansel H. C. Differences in results for aneurysm vs occlusive disease after bifurcation grafts: results of 100 elective grafts. Arch Surg. 1980 Oct;115(10):1173–1175. doi: 10.1001/archsurg.1980.01380100023005. [DOI] [PubMed] [Google Scholar]
- Tremble P. M., Damsky C. H., Werb Z. Fibronectin fragments, but not intact fibronectin, signalling through the fibronectin receptor induce metalloproteinase gene expression in fibroblasts. Matrix Suppl. 1992;1:212–214. [PubMed] [Google Scholar]
- Tromp G., Wu Y., Prockop D. J., Madhatheri S. L., Kleinert C., Earley J. J., Zhuang J., Norrgård O., Darling R. C., Abbott W. M. Sequencing of cDNA from 50 unrelated patients reveals that mutations in the triple-helical domain of type III procollagen are an infrequent cause of aortic aneurysms. J Clin Invest. 1993 Jun;91(6):2539–2545. doi: 10.1172/JCI116490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wart H. E., Birkedal-Hansen H. The cysteine switch: a principle of regulation of metalloproteinase activity with potential applicability to the entire matrix metalloproteinase gene family. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5578–5582. doi: 10.1073/pnas.87.14.5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vine N., Powell J. T. Metalloproteinases in degenerative aortic disease. Clin Sci (Lond) 1991 Aug;81(2):233–239. doi: 10.1042/cs0810233. [DOI] [PubMed] [Google Scholar]
- Welgus H. G., Campbell E. J., Cury J. D., Eisen A. Z., Senior R. M., Wilhelm S. M., Goldberg G. I. Neutral metalloproteinases produced by human mononuclear phagocytes. Enzyme profile, regulation, and expression during cellular development. J Clin Invest. 1990 Nov;86(5):1496–1502. doi: 10.1172/JCI114867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welgus H. G., Connolly N. L., Senior R. M. 12-o-Tetradecanoyl-phorbol-13-acetate-differentiated U937 cells express a macrophage-like profile of neutral proteinases. High levels of secreted collagenase and collagenase inhibitor accompany low levels of intracellular elastase and cathepsin G. J Clin Invest. 1986 May;77(5):1675–1681. doi: 10.1172/JCI112485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welgus H. G., Stricklin G. P. Human skin fibroblast collagenase inhibitor. Comparative studies in human connective tissues, serum, and amniotic fluid. J Biol Chem. 1983 Oct 25;258(20):12259–12264. [PubMed] [Google Scholar]
- Woessner J. F., Jr Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J. 1991 May;5(8):2145–2154. [PubMed] [Google Scholar]