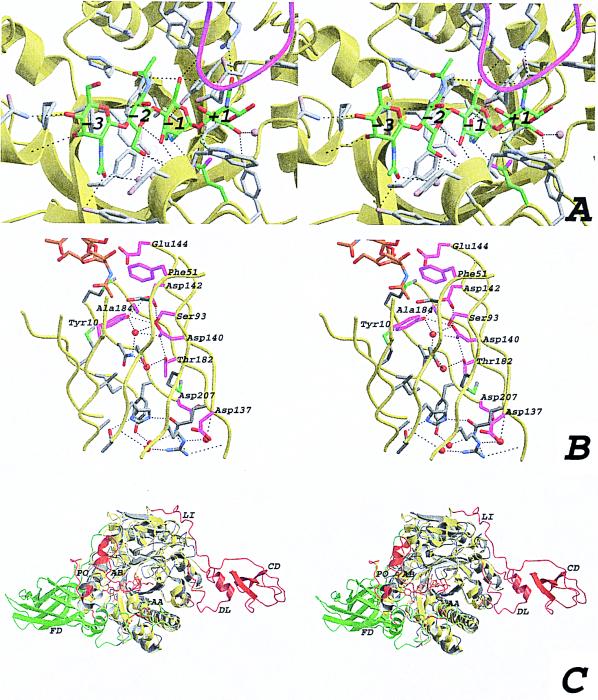
Figure 3.
(A) Stereo view of the active site with the modeled chitotetraose (same view as in Fig. 1C). The ChiB backbone is shown as a yellow ribbon. The modeled chitotetraose is shown in a stick representation, with the carbons colored green. Side chains within 5 Å of the chitotetraose are depicted by gray sticks, and also are indicated in Fig. 1. Possible hydrogen bonds are drawn as black dashed lines, and the residues involved are indicated in Fig. 1. The four water molecules that are predicted to be replaced by the substrate are shown as blue transparent spheres. The GlcNAc residues are labeled from −3 to +1, corresponding to their location with respect to the active site residue (15). The loop around residue 316, partially covering the active site, is shown in magenta. (B) Stereo view of the interior of the ChiB TIM barrel. The strands forming the TIM barrel are shown as a yellow ribbon. Side chains of residues lining the inside of the barrel are shown as sticks. Side chains conserved in ChiA, ChiB, and hevamine are colored magenta. Water molecules in the structure are shown as red spheres. Hydrogen bonds are shown as black dashed lines. Conserved residues are labeled according to the ChiB sequence. Part of the chitotetraose model is shown as sticks, with carbon atoms colored orange. (C) Stereo view of a superposition of ChiA and ChiB. Both structures are shown in a ribbon representation. ChiB is colored yellow, except for residues that correspond to insertions in ChiB with respect to ChiA, which are colored red. ChiA is colored gray except for residues that correspond to insertions in ChiA with respect to ChiB, which are colored green. Some insertions are indicated with two-letter labels. AA, active site covering loop in ChiA; AB, active site covering loop in ChiB; CD, ChBD in ChiB; DL, ChBD support loop in ChiB; FD, fibronectin domain in ChiA; LI, linker in ChiB; PO, porch loop in ChiB.