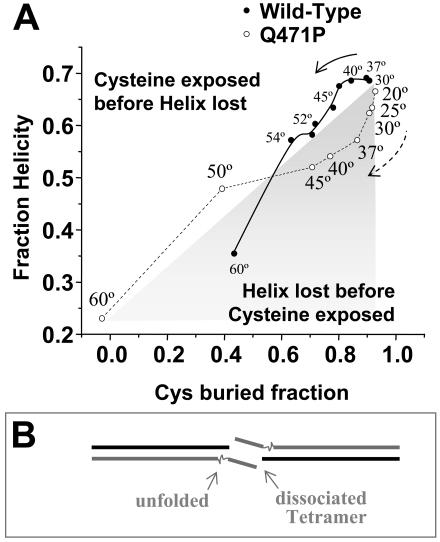
Figure 6.
Proline mutation alters folding state. (A) Plot of helicity compared with fraction unlabeled cysteine at temperatures ranging from 20°C to 60°C reveals divergent unfolding pathways on mutation (B) The reduced structural stability of the mutant is speculated to add flexibility and to increase entropy in destabilizing the αβassociation in the tetramer.