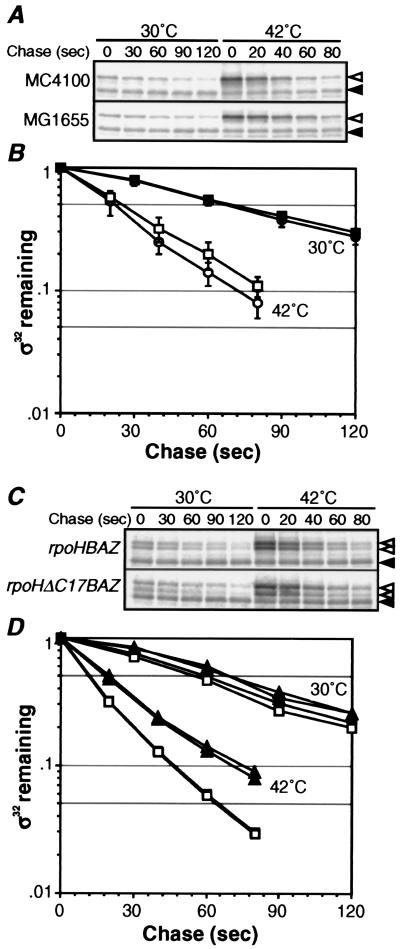
Figure 4.
Differential stability of σ32 at 30°C and 10 min after shift to 42°C. (A and C) SDS/PAGE patterns of σ32 remaining in pulse-chase experiments. Cells were grown at 30°C, shifted to 42°C, and portions taken at t = 0 and 10 min were pulse labeled with [35S]Met (1,200 Ci/mmol, 200 μCi/ml) for 30 s, and chased with excess unlabeled Met for 30 or 20 s at 30 or 42°C, respectively, and set as t = 0. Aliquots were then taken at the times indicated, and σ32 (◃) and σ32-B or σ32ΔC17 (hatched arrowheads) were immunoprecipitated and analyzed by SDS/PAGE as in Fig. 2A. (◂) Reference as in Fig. 2. (B and D) Quantification of protein stability. (B) ●, MC4100, 30°C; ○, MC4100, 42°C; ■, MG1655, 30°C; and □, MG1655, 42°C. (D) □, σ32-B in MC4100 (λrpoHBAZ); ■, σ32 in MC4100 (λrpoHBAZ); ▵, σ32ΔC17 in MC4100 (λrpoHΔC17BAZ); and ▴, chromosomally encoded σ32 in MC4100 (λrpoHΔC17BAZ).