Figure 6.
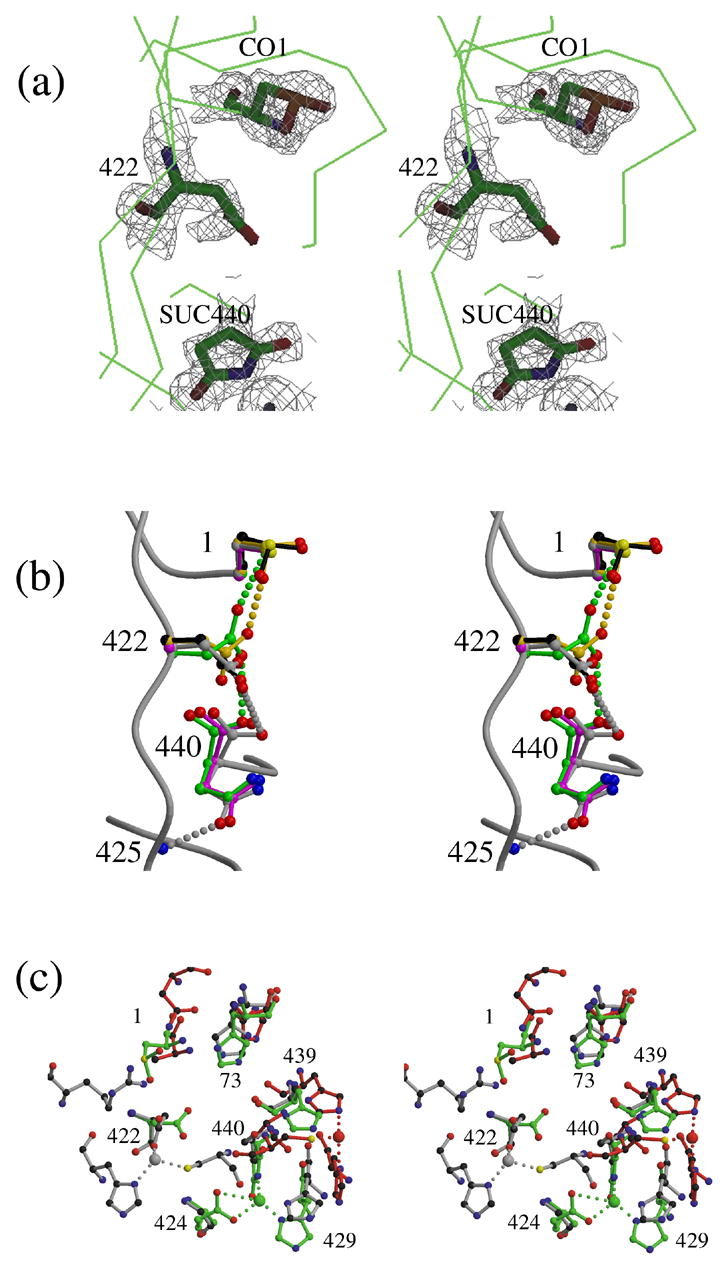
Structural comparisons of the intein N-termini and C-termini of inteins. (a) Model and electron density (contoured at 1.5 σ) of residues 1, 422 and 440 in the structure of ΔI-SM (molecule A), showing observed modifications: CO1: cysteine sulfinic acid; SUC440: aminosuccinimide. Despite inconsistent electron density, residue 422 is included in the model for I-SM as an Asp because the modification of the residue has not been characterized. (b). Conformations of Cys1, Asp422, and Asn440 in the structures of ΔΔIhh-SM (grey), ΔΔIhh-CM (magenta) and ΔΔIhh (green) and of CO1 and Asp422 in ΔI-SM molecules A (black) and B (gold). (c). Comparison of the zinc-binding sites observed in the structures of three different inteins: ΔI-SM (green), PI-SceI34 (red) and dnaE17 (grey). Residue numbers shown correspond to those of ΔI-SM.
