Abstract
Aquatic organisms often detect predators via water-borne chemical cues, and respond by showing reduced activity. Prey responses may be correlated with the concentration of predation cues, which would result in graded antipredator behavioral responses that adjust potentially costly behavioral changes to levels that are commensurate with the risk of predation. Larvae of the predatory mosquito Toxorhynchites rutilus prey upon other container-dwelling insects, including larvae of the mosquito Ochlerotatus triseriatus. Previous work has established that O. triseriatus reduce movement, foraging, and time below the surface, and increase the frequency of resting at the surface, in the presence of water-borne cues from predation by T. rutilus. We tested whether these responses by O. triseriatus are threat sensitive by recording behavior of fourth instar larvae in two runs of an experiment in which we created a series of concentrations (100, 10, 1, 0.1, and 0.01% and 100, 70, 40, 20, and 10%) of water that had held either O. triseriatus larvae alone (control) or a T. rutilus larva feeding on O. triseriatus (predation). We also tested whether associated effects on time spent feeding are threat sensitive by determining whether frequencies of filtering or browsing are also related to concentration of cues. The frequencies of resting and surface declined, whereas frequency of filtering (but not browsing) increased more rapidly with a decrease in concentration of predation cues compared with control cues. Thus, O. triseriatus shows a threat sensitive behavioral response to water-borne cues from this predator, adjusting its degree of behavioral response to the apparent risk of predation.
Introduction
Antipredator behavior is postulated to be costly because it often reduces the time and energy that can be devoted to other important activities like foraging (Relyea & Werner 1999; Van Buskirk 2000; Relyea 2001; Stoks et al. 2005). The threat sensitivity hypothesis states that prey will alter their predation avoidance responses according to the magnitude of the threat, so that as predation risk increases, time spent on predation avoidance behavior will increase (Helfman 1989). Evidence supporting the threat sensitivity hypothesis exists for a number of systems in which prey respond more strongly to more predators, to more dangerous predators, or to more risky situations (e.g. Bishop & Brown 1992; Scarratt & Godin 1992; Peckarsky 1996; Puttlitz et al. 1999; Chivers et al. 2001; Smith & Belk 2001; Kusch et al. 2004; Laurila et al. 2004; Mirza et al. 2006). Aquatic prey often detect the presence of a predator via water-borne chemical cues, and react by showing reduced activity, including reduced foraging (e.g. Sih 1987; Lima & Dill 1990; Chivers et al. 1996; Lima 1998; Wisenden 2000; Kusch et al. 2004). Behavioral responses that are correlated with concentrations of these water-borne cues may be important if different levels of antipredator responses (Laurila 2000; Kusch et al. 2004) adjust costly behavioral changes to levels that are commensurate with threat of predation.
Larvae of the mosquito Ochlerotatus triseriatus (Say) develop in water-filled tree holes and similar man-made containers. Larval O. triseriatus often co-occur with larvae of the predatory mosquito Toxorhynchites rutilus (Coquillet), which are ambush predators (Steffan & Evenhuis 1981) feeding on other aquatic insects, including immature O. triseriatus. Ochlerotatus triseriatus oviposit on the sides of the container above the water line and the eggs hatch when the water level rises due to rainfall. Toxorhynchites rutilus adult females oviposit on the surface of the water and their eggs hatch in approx. 24 h (Steffan & Evenhuis 1981). Ochlerotatus triseriatus larvae can reach pupation in 12–14 d under favorable conditions (Hechtel & Juliano 1997) whereas T. rutilus larvae will take approx. 16–20 d (Steffan & Evenhuis 1981). Ochlerotatus triseriatus alters its behavior in response to water-borne cues from predation by T. rutilus, becoming less active, reducing foraging, and moving away from the bottom of the container (Juliano & Gravel 2002; Kesavaraju & Juliano 2004). These changes appear to lessen risk of predation (Juliano & Reminger 1992; Grill & Juliano 1996). Ochlerotatus triseriatus do not show any change in behavior when held outside of cages containing feeding T. rutilus (Hechtel & Juliano 1997). Ochlerotatus triseriatus adopt low-risk behavior in water with crushed conspecifics (unpubl. data) and in waters with the remnants of the act of predation including solid particulate matter (Juliano & Gravel 2002; Kesavaraju & Juliano 2004). These results suggest that the cues to which they respond may include chemicals released from the act of predation, and that solid residues from predation are important for their response. Whether this behavioral response and associated costs in reduced foraging are threat sensitive to different concentrations of cues from T. rutilus predation is the question we address in this paper.
Methods
The O. triseriatus larvae that we used in this experiment were descendants of individuals we collected from tree holes at Parklands Merwin Reserve, north of Normal, IL, USA. Field collected larvae were reared to adulthood and were blood fed with anaesthetized guinea pigs (IACUC protocol 01-2005) to obtain F1 eggs. The T. rutilus larvae that we used in this experiment were from a laboratory colony that originated in Vero Beach, FL, USA. Field collected T. rutilus larvae were added to the colony from time to time.
We have shown that behavioral responses of O. triseriatus larvae to T. rutilus predation can be quantified by observing larvae in 50 ml cups with 50 ml of water (Kesavaraju & Juliano 2004). In such experiments predation water was prepared by feeding a T. rutilus larva with 10 O. triseriatus larvae per day for up to 5 d; whereas control water was prepared by rearing 10 O. triseriatus larvae alone for a similar period (Kesavaraju & Juliano 2004). In both cases, all predator and prey larvae were removed from the prepared water before testing behavioral responses of naive test larvae (Kesavaraju & Juliano 2004). We use this protocol in the current experiments. Behavior of fourth instar larvae (1-d post-molt) was recorded in different dilutions of predation and control water. The standard water preparations were designated as 100% concentration of any water-borne cues to predation risk. We manipulated concentrations of cues by diluting water from those standard preparations with different amounts of deionized (DI) water. We then observed the behavior of O. triseriatus larvae in these different concentrations. Our experiment was divided into two separate runs, each spanning a different range of dilutions of the treatment water. Apart from the concentrations, we used the same procedures for both runs of this experiment.
Run 1
For the predation water treatment, one T. rutilus third instar larva (approx. 10–12-mm long) was held for 5 d with 10 fourth instar O. triseriatus (approx. 7–10-mm long) in 100 ml plastic tripour beakers with 60 ml of water. For control water, 10 fourth instar O. triseriatus were held in 100 ml plastic tripour beakers with 60 ml of water for 5 d. In both treatments, eaten, dead, or pupated larvae were replaced daily. Prior to the experiment we removed all larvae (prey and predator) from the prepared water and homogenized any remaining particulate material (e.g. predator feces and prey feces) in each replicate using a Tissue Tearor® (Biospec products, Bartlesville Oklahoma, USA). We divided the 60 ml of each replicate into 50, 5, 0.5, 0.05, and 0.005-ml aliquots and added 0, 45, 49.5, 49.95, and 49.995 ml, respectively, of DI water to make concentrations of 100, 10, 1, 0.1, and 0.01% of the original treatment water.
Run 2
In the first run we used low concentrations (10, 1, 0.1, and 0.01%) and although there was a clear difference between the 100% concentration and the lower concentrations, the behavioral responses among the lower levels were indistinguishable (see Fig. 1a, b). So we conducted the second run using higher concentrations. We prepared 20 predation (60-ml water, 10 O. triseriatus + 1 T. rutilus held for 5 d) and 20 control (60 ml water, 10 O. triseriatus held for 5 d) batches of water as in run 1. After preparing the water, we removed remaining predator and prey larvae, combined two randomly chosen replicates within a treatment, and homogenized solid matter with a Tissue Tearor®. This procedure resulted in 10 control and 10 predation water replicates of 120-ml each. We divided the 120 ml from each replicate into 50, 35, 20, 10, and 5 ml and added 0, 15, 30, 40, and 45 ml of DI water to make concentrations of 100, 70, 40, 20, and 10% of the original treatment water. In run 1 the highest concentration we used after 100% was 10%, whereas the lowest concentration in run 2 was 10%. The two runs combined enable us to test for graded behavioral responses across the full range of concentration of cues.
Fig. 1.
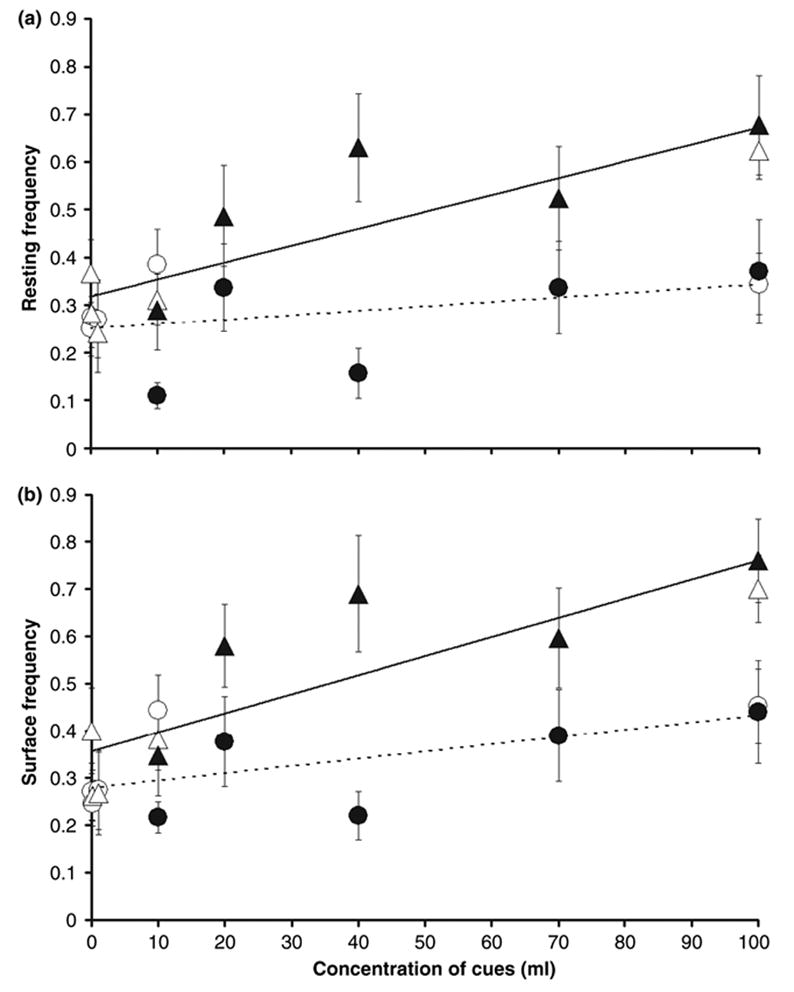
(a) Means (±SE; total number of larvae observed per concentration n = 8–10 each) of resting frequency for each treatment-concentration combination, plotted against the concentration of cues for both runs combined. (b) Means (±SE; n = 8–10 larvae each) of surface frequency for each treatment-concentration combination, plotted against the concentration of cues for both runs combined. Triangles = predation; circles = control. Open symbols = run 1; Closed symbols = run 2. Dotted trend lines = control treatments; solid trend lines = predation treatments. The points that appear to be on the vertical axis for both resting and surface range from 0.01% to 1%. The open figures which represent run 1 are clustered near the origin of the graph, except for one point at 100% concentration. The points of the two runs overlap only at 100% and 10% concentrations. The equations are: resting control y = 0.0009x + 0.0251 (R2 = 0.1702), resting predation y = 0.0035x + 0.3192 (R2 = 0.7582). Surface control y = 0.0015x + 0.279 (R2 = 0.4163), surface predation y = 0.0040x + 0.3569 (R2 = 0.7553)
For both runs, we hatched larvae for behavioral comparisons from F1 eggs using nutrient broth (0.3 mg/750 ml). We reared larvae individually in 18-ml glass vials with 5 ml of water. We fed larvae with liver powder suspension (LPS) prepared by adding 0.3-g liver powder in 1000 ml of DI water (Juliano & Gravel 2002). We held the LPS on a magnetic stir plate and pipetted standard amounts of LPS to each larva while the suspension was stirred to ensure homogeneous delivery of food. We gave first-day larvae 0.5 ml of the LPS. We gave third-day larvae 1 ml of LPS, and repeated this every other day until they reached the fourth instar. We then isolated fourth instar larvae for 24 h in 50-ml disposable polystyrene cups (Diameter, top – 60 mm, bottom –30 mm; height – 45 mm) with 50 ml of DI water before transferring them to the treatment water for behavior recording.
Video Recording
We recorded the behavior of the larvae in predation and control water using a video camera and a computer. We used a WinFast XP® 2000 PCI card (Leadtek, Fremont, California, USA) and its associated software to record MPEG-2 format video. We allowed experimental larvae to acclimate to their containers for 10 min prior to beginning recording. Each video clip was 30-min long with only six treatment cups in view because of resolution constraints. We allocated replicates to observation periods so that each clip included both control and predation water preparations.
Behavioral Data
Among the activities and positions of larvae in containers, resting and surface are the least likely to lead to predation (Juliano & Reminger 1992). We have shown that O. triseriatus larvae spend more time resting at the surface when they are in water with T. rutilus predation cues (Juliano & Gravel 2002; Kesavaraju & Juliano 2004). Browsing and filtering are the foraging behaviors of mosquitoes (Merritt et al. 1992; Yee et al. 2004). So, larvae responding to the threat in the environment by reducing movement will have less time to forage. If larvae show threat sensitive behavioral responses, their foraging activity should vary with the level of threat in the environment. Thus, upon playback of video clips, we recorded whether larvae were resting, browsing, filtering, or at the surface of the containers every minute for 30 min.
Statistical Analysis
We converted counts of resting, browsing, filtering, and surface into proportions (out of 30, at one behavioral observation per minute for 30 min) for each larva. We analyzed these proportions by comparing linear regressions using a mixed model analysis (PROC MIXED, SAS Institute Inc. 1990). Proportions of resting, surface, filtering, and browsing were dependent variables. Manipulated concentration of cues and our two treatments (control, predation water) were independent variables. We combined both runs in one analysis, including a categorical variable to account for any differences in the responses of larvae between runs. We estimated the slopes using linear models with concentration as a continuous variable and treatment, replicate (a random effect) nested in treatment and run. We used an ANCOVA approach to test for differences in the slopes of the regressions for predation and control treatments with the different concentrations as the covariate. Such a difference would yield a significant treatment by concentration interaction (SAS Institute Inc. 1990). Tests of the treatment main effect used the random effect of replicate nested in treatment and run as the denominator of the F test, as appropriate for a model with random nested effects (Potvin 2001). All other effects were tested against error. We also tested whether the slopes of regressions of each behavior vs. concentration (within either predation or control treatments) were different from zero.
Results
Antipredator Behavior
There were significant treatment by concentration interactions for both resting and surface (Table 1), indicating that the slopes vs. concentration were significantly different for the two treatments, as expected. In predation water, frequencies of both resting (t144 = 5.02, p = <0.0001) and surface (t144 = 5.60, p = <0.0001) showed significant increases as concentration increased (Fig. 1a, b, dashed lines). In control water (Fig. 1a, solid lines), frequency of resting yielded a slope that was not significantly different from zero (t144 = 1.50, p = 0.1349). For surface, the slope of control was significantly >0 (t144 = 2.30, p = 0.0229), but also significantly lower than the slope for predation (Table 1, Fig. 1b).
Table 1.
Regression of frequency of resting, surface, browsing, and filtering vs. concentration for both runs of the experiment combined
| Variable
|
Resting
|
Surface
|
Browsing
|
Filtering
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Source | Num df | Den df | F | p | F | p | F | p | F | p |
| Treatment | 1 | 36 | 2.09 | 0.1564 | 2.49 | 0.1234 | 0.96 | 0.3332 | 0.89 | 0.3511 |
| Concentration | 1 | 144 | 21.30 | <0.0001 | 31.24 | <0.0001 | 0.00 | 0.9603 | 37.96 | <0.0001 |
| Treatment × concentration | 1 | 144 | 6.20 | 0.0139 | 5.46 | 0.0208 | 0.85 | 0.3582 | 6.86 | 0.0098 |
Effects that are significant at α = 0.05 are highlighted in boldface. The random effect of replicate (treatment run) was used as the denominator of the F test for the treatment effect; error was used as the denominator of the F tests for other effects.
Foraging Behavior
None of the effects were significant for browsing and the slopes of control and predation water were not significantly different from zero (Table 1, Fig. 2a). Treatment by concentration interaction was significant for filtering (Table 1). Frequency of filtering decreased significantly with increase in concentration of predation water (Fig. 2b). Slope for filtering in predation water was significantly <0 (t144 =−6.21, p = <0.0001). In control water, the slope was also significantly <0 (t144 = −2.50, p = 0.0134) but also significantly lower in magnitude than the corresponding slope in predation water (Table 1, Fig. 2b)
Fig. 2.
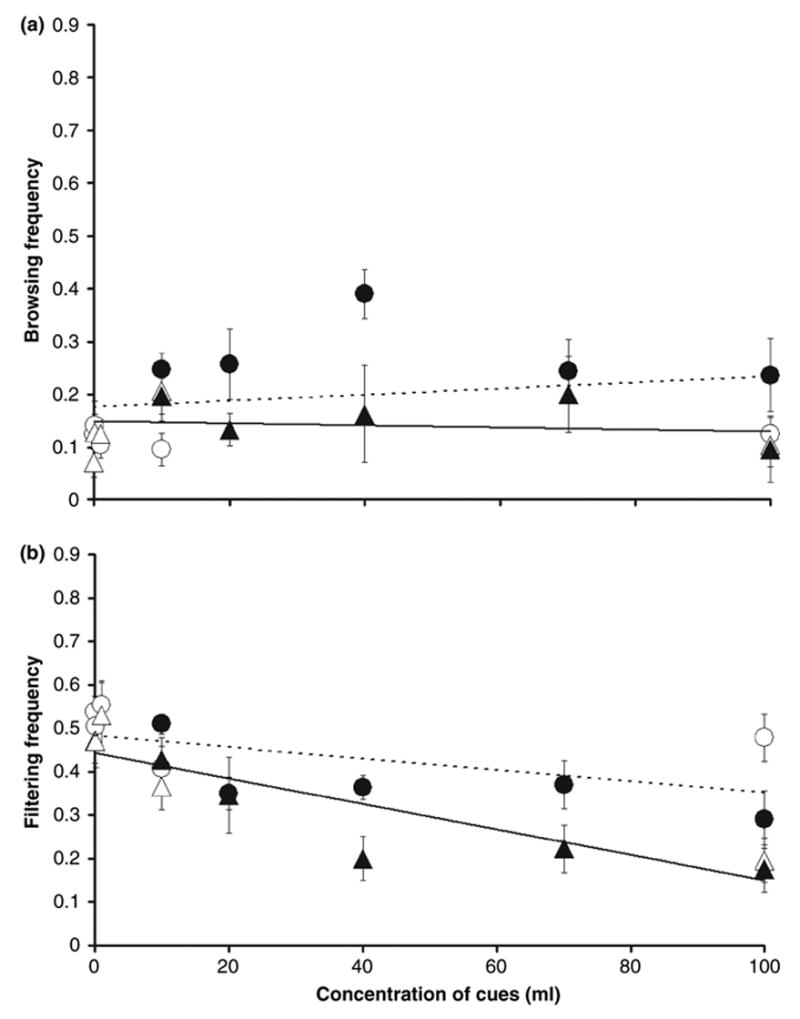
(a) Means (±SE; n = 8–10 larvae each) of browsing frequency for treatment-concentration combinations, plotted against the concentration of cues for both runs combined. (b) Means (±SE; n = 8–10 larvae each) of filtering frequency for treatment-concentration combinations, plotted against the concentration of cues for both runs combined. Triangles = predation; circles = control. Open Symbols = run 1; Closed Symbols = run 2. Dotted trend lines = control treatments; solid trend lines = predation treatments. The points that appear to be on the vertical axis for both resting and surface range from 0.01% to 1%. The open figures which represent run 1 are clustered near the origin of the graph except for one point at 100% concentration. The points of the two runs overlap only at 100% and 10% concentrations. The equations are: browsing control y = 0.0012x + 0.1764 (R2 = 0.0624), browsing predation y = −0.0004x + 0.1489 (R2 = 0.0236). Filtering control y = −0.0013x + 0.4822 (R2 = 0.3328), filtering predation y = −0.0029x + 0.4435 (R2 = 0.7973)
Discussion
As water-borne chemical cues to predation are diluted, O. triseriatus is capable of adjusting its degree of behavioral response to the apparent risk of predation. As in other species subject to predation, these mosquitoes have the flexibility to adopt antipredator behavior corresponding to the intensity of threat in their environment (Helfman 1989; Bishop & Brown 1992; Scarratt & Godin 1992; Peckarsky 1996; Smith & Belk 2001; Kusch et al. 2004; Laurila et al. 2004; Mirza et al. 2006). Although allocation of time and energy toward antipredator behavior is essential, it appears to come at the expense of reduced foraging effort, specifically reduced filtering. Reduced filtering could have a cost in reduced growth and development, as in other systems (Sih 1984; Relyea & Werner 1999), but those costs have not yet been quantified in this mosquito system.
Mosquitoes forage by both browsing surfaces and filtering from the water column and O. triseriatus has been described as foraging predominantly by browsing (Merritt et al. 1992). Yee et al. (2004) showed that O. triseriatus alter their foraging behavior depending on the nature of food available in the environment. Larvae spend more time filtering in water with suspended particulate food and more time browsing in water with food primarily available on surfaces (Yee et al. 2004). The treatment cups in our experiment would have primarily had food (bacteria, homogenized particulate matter) suspended in the water column, so that larvae would be expected mainly to forage by filtering, and this is what is observed in control water and in dilute predator water (see Fig. 2b). Because filtering is the dominant foraging mode in this environment, effects of dilution of predator-derived cues primarily affect frequency of filtering. Increased filtering by larvae with decreased cues to predation risk suggests that O. triseriatus larvae minimize the costs of reduced foraging when the perceived threat of predation is low. The lack of a significant effect of dilution of water-borne cues on browsing (Table 1, Fig. 2a) probably results from browsing being rare in this experimental setting. Adopting low predation risk behavior (i.e. resting at the surface, with low filtering) in low-risk situations or showing constant low predation risk behavior in the absence of predators may lower the fitness of O. triseriatus larvae by imposing costs of reduced growth and development, but yielding little or no predator avoidance benefit. Previous studies on the response of O. triseriatus in water with predation cues have quantified multiple activities and positions exhibited by mosquitoes (Grill & Juliano 1996; Juliano & Gravel 2002; Kesavaraju & Juliano 2004). Among the activities (resting, filtering, browsing, and thrashing), resting is typically negatively correlated with foraging behaviors of browsing or filtering. Our current study suggests that shifting to the safer behavior of resting (Juliano & Reminger 1992) specifically reduces allocation of effort to filtering, probably the most productive foraging mode in the experimental environment.
In nature, multiple T. rutilus can sometimes be found in some tree holes and man-made containers, but these predators are highly cannibalistic and typically only one survives to pupation in a container (Steffan & Evenhuis 1981). Behavioral responses of O. triseriatus to concentrations of water-borne cues from predation could be advantageous because higher concentrations of cues indicate the presences of more predators. Containers in nature vary in water volume, and with lower volume, concentration of cues from even one predator would presumably be higher than in a larger volume. Higher concentration in this context could indicate greater probability of encountering the predator within the small volume. Kusch et al. (2004) suggested that concentration of predator cues could indicate proximity of a predator. If this is true for these tree hole systems, it would require that water mixing is sufficiently limited that gradients of predator-derived cues can develop. We have no data on this subject, but the small size and lack of flow in most tree holes suggests that such gradients are possible.
According to the threat sensitive hypothesis, the intensity of prey response is dependent on the intensity of threats present in the environment (Helfman 1989). The means by which prey detect gradients of threat varies among species and systems. Prey may respond more to a combination of a predator cue and an injured conspecific cue, and less to either injured conspecifics or predator cues alone (e.g. Keppel & Scrosati 2004). Prey may respond more to the actual consumption of conspecifics than to the presence of dead conspecifics (Murray & Jenkins 1999). In other cases, prey respond more to conspecific vs. heterospecific predation cues (e.g. Mirza et al. 2006), or respond more to greater concentrations of chemical cues (e.g. Kusch et al. 2004). In O. triseriatus, water-borne cues from predation by T. rutilus on either conspecifics or an ecologically similar species (Aedes albopictus) are equally likely to trigger antipredator responses (Kesavaraju & Juliano 2004). In contrast, the physical presence of the predator T. rutilus, including visual and tactile stimuli, does not induce a similar shift to low risk behavior (Hechtel & Juliano 1997). Also, O. triseriatus behavioral responses do not differ significantly between predation cues from the actual consumption of O. triseriatus by T. rutilus and the mere crushing of con-specifics or crushing of another insect (unpubl. data). These results suggest that O. triseriatus senses risk of predation from some non-species specific cue, such as insect body parts or fluids, that are released into the environment via predation. They might be responding to unidentified cues from specific sources (e.g. specialized cells), as is the case for behavioral responses of some fishes (Wisenden 2000).
Graded threat sensitive responses seem to be common in freshwater systems, but the cues involved are diverse. A similar experiment using range of dilutions of chemical cues (filtered homogenate of ground skin of conspecifics, diluted to different concentrations using distilled water) in northern redbelly dace also found a positive correlation between intensity of antipredator response and concentration of cues (Dupuch et al. 2004). Studies with brook charr (Mirza & Chivers 2003a) suggest that predator diet can affect prey responsiveness and that some species assess the level of risk based on a predator’s recent consumption. Larvae of threespine sticklebacks assessed apparent predation risk based on the size of a conspecific predator and shifted their level of behavioral response accordingly (Bishop & Brown 1992). Mirza & Chivers (2003b) showed that rainbow trout exposed to chemical cue concentrations below the level that produces an observable behavioral change nonetheless had increased survival in the presence of a predator. This result indicates that behavioral responses of prey to low concentrations of cues may not be always evident to an observer.
Behavioral studies of antipredator responses focus mainly on the change in prey behavior in the presence vs. absence of a predator or of predator-derived cues (Sih et al. 2000). Threat sensitive, graded responses to apparent risk can provide information on costs and benefits of prey behavioral changes, and on how prey balance trade offs in allocation of time and energy. Such trade offs are likely to affect individual foraging and growth, and may thus be important for understanding, how behavior of individuals is translated into ecological effects of predators on populations and communities.
Acknowledgments
We thank L.P. Lounibos, B.W. Alto, and R. Escher of Florida Medical Entomology Laboratory, University of Florida, Vero Beach, Florida for providing T. rutilus and laboratory space, and two anonymous referees for helpful comments. This research was supported by grants from the National Institute of Allergy and Infectious Disease (R01 AI-44793, Illinois State University subcontract) and Illinois State University to SAJ.
References
- Bishop TD, Brown JA. Threat-sensitive foraging by larval threespine sticklebacks (Gasterosteus aculeatus) Behav Ecol Sociobiol. 1992;31:133–133. [Google Scholar]
- Chivers DP, Wisenden BD, Smith RJF. Damselfly larvae learn to reconize predators from chemical cues in the predator’s diet. Anim Behav. 1996;52:315–315. [Google Scholar]
- Chivers DP, Mirza RS, Bryer PJ, Kiesecker JM. Threat-sensitive predator avoidance by slimy sculpins: understanding the importance of visual versus chemical information. Can J Zool. 2001;79:867–867. [Google Scholar]
- Dupuch A, Magnan P, Dill LM. Sensitivity of northern redbelly dace, Phoxinus eos, to chemical alarm cues. Can J Zool. 2004;82:407–407. [Google Scholar]
- Grill CP, Juliano SA. Predicting species interactions based on behaviour: predation and competition in container-dwelling mosquitoes. J Anim Ecol. 1996;65:63–63. [Google Scholar]
- Hechtel LJ, Juliano SA. Effects of a predator on prey metamorphosis: plastic responses by prey or selective mortality? Ecology. 1997;78:838–851. [Google Scholar]
- Helfman GS. Threat-sensitive predator avoidance in damselfish-trumpetfish interactions. Behav Ecol Sociobiol. 1989;24:47–47. [Google Scholar]
- Juliano SA, Gravel ME. Predation and the evolution of prey behavior: an experiment with tree hole mosquitoes. Behav Ecol. 2002;13:301–301. [Google Scholar]
- Juliano SA, Reminger L. The relationship between vulnerability to predation and behavior of larval tree-hole mosquitoes: geographic and ontogenetic differences. Oikos. 1992;63:465–467. [Google Scholar]
- Keppel E, Scrosati R. Chemically mediated avoidance of Hemigrapsus nudus (Crustacea) by Littorina scutulata (Gastropoda): effects of species coexistence and variable cues. Anim Behav. 2004;68:915–915. [Google Scholar]
- Kesavaraju B, Juliano SA. Differential behavioral responses to water-borne cues to predation in two container-dwelling mosquitoes. Ann Entomol Soc Am. 2004;97:194–194. doi: 10.1603/0013-8746(2004)097[0194:dbrtwc]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusch RC, Mirza RS, Chivers DP. Making sense of predator scents: investigating the sophistication of predator assessment abilities of fathead minnows. Behav Ecol Sociobiol. 2004;55:551–551. [Google Scholar]
- Laurila A. Behavioural responses to predator chemical cues and local variation in antipredator performance in Rana temporaria tadpoles. Oikos. 2000;88:159–168. [Google Scholar]
- Laurila A, Jarvi-Laturi M, Pakkasmaa S, Merila J. Temporal variation in predation risk: stage-dependency, graded responses and fitness costs in tadpole antipredator defenses. Oikos. 2004;107:90–99. [Google Scholar]
- Lima SL. Stress and decision making under the risk of predation: recent developments from behavioral, reproductive, and ecological perspectives. Adv Stud Behav. 1998;27:215–290. [Google Scholar]
- Lima SL, Dill LM. Behavioral decisions made under the risk of predation: a review and prospectus. Can J Zool. 1990;68:619–619. [Google Scholar]
- Merritt RW, Dadd RH, Walker ED. Feeding behavior, natural food, and nutritional relationships of larval mosquitoes. Annu Rev Entomol. 1992;37:349–349. doi: 10.1146/annurev.en.37.010192.002025. [DOI] [PubMed] [Google Scholar]
- Mirza RS, Chivers DP. Predator diet cues and the assessment of predation risk by juvenile brook charr: do diet cues enhance survival? Can J Zool. 2003a;81:126–132. [Google Scholar]
- Mirza RS, Chivers DP. Response of juvenile rainbow trout to varying concentrations of chemical alarm cue: response thresholds and survival during encounters with predators. Can J Zool. 2003b;81:88–95. [Google Scholar]
- Mirza RS, Mathis A, Chivers DP. Does temporal variation in predation risk influence the intensity of anti-predator responses? A test of the risk allocation hypothesis. Ethology. 2006;112:44–51. [Google Scholar]
- Murray DL, Jenkins CL. Perceived predation risk as a function of predator dietary cues in terrestrial salamanders. Anim Behav. 1999;57:33–33. doi: 10.1006/anbe.1998.0986. [DOI] [PubMed] [Google Scholar]
- Peckarsky BL. Alternative predator avoidance syndromes of stream-dwelling mayfly larvae. Ethology. 1996;77:1888–1905. [Google Scholar]
- Potvin C. ANOVA: experimental layout and analysis. In: Scheiner SM, Gurevitch J, editors. Design and Analysis of Ecological Experiments. 2. Oxford Univ. Press; New York: 2001. pp. 63–76. [Google Scholar]
- Puttlitz MH, Chivers DP, Kiesecker JM, Blaustein AR. Threat-sensitive predator avoidance by larval pacific treefrogs (Amphibia, Hylidae) Ethology. 1999;105:449–456. [Google Scholar]
- Relyea RA. Morphological and behavioral plasticity of larval anurans in response to different predators. Ecology. 2001;82:523–540. [Google Scholar]
- Relyea RA, Werner EE. Quantifying the relation between predator-induced behavior and growth performance in larval anurans. Ecology. 1999;80:2117–2124. [Google Scholar]
- SAS Institute Inc. SAS/STAT Users Guide, Version 6. 4. SAS Institute Inc.; Cary, NC: 1990. [Google Scholar]
- Scarratt AM, Godin J-GJ. Foraging and anti-predator decisions in the hermit crab Pagurus acadianus (Benedict) J Exp Mar Biol Ecol. 1992;156:225–225. [Google Scholar]
- Sih A. The behavioral response race between predator and prey. Am Nat. 1984;123:143–150. [Google Scholar]
- Sih A. Predator and prey lifestyles: an evolutionary and ecological overview. In: Kerfoot WC, Sih A, editors. Predation: Direct and Indirect Impacts on Aquatic Communities. Univ. Press of New England; Hanover: 1987. pp. 203–224. [Google Scholar]
- Sih A, Ziemba R, Harding KC. New insights on how temporal variation in predation risk shapes prey behavior. Trends Ecol Evol. 2000;15:3–3. doi: 10.1016/s0169-5347(99)01766-8. [DOI] [PubMed] [Google Scholar]
- Smith ME, Belk MC. Risk assessment in western mosquitofish (Gambusia affinis): do multiple cues have additive effects? Behav. Ecol Sociobiol. 2001;51:101–101. [Google Scholar]
- Steffan WA, Evenhuis NL. Biology of Toxorhynchites. Annu Rev Entomol. 1981;26:159–159. [Google Scholar]
- Stoks R, DeBlock M, Van de Meutter F, Johansson F. Predation cost of rapid growth: behavioural coupling and physiological decoupling. J Anim Ecol. 2005;74:708–708. [Google Scholar]
- Van Buskirk J. The costs of an inducible defense in anuran larvae. Ecology. 2000;81:2813–2821. [Google Scholar]
- Wisenden BD. Olfactory assessment of predation risk in the aquatic environment. Philos Trans R Soc Lond B Biol Sci. 2000;355:1205–1205. doi: 10.1098/rstb.2000.0668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee DA, Kesavaraju B, Juliano SA. Larval feeding behavior of three co-occuring species of container mosquitoes. J Vector Ecol. 2004;29:315–315. [PMC free article] [PubMed] [Google Scholar]


