ABSTRACT
The expanded endonasal approach provides access to the entire ventral skull base for resection of neoplasms involving the skull base and brain. The creation of large defects of the bone and dura endoscopically presents unique reconstructive challenges. A layered reconstruction of the dura with inlay and onlay fascial grafts covered with fat grafts is an effective technique for repair. An intranasal balloon catheter is used to provide counterpressure in the early phase of healing and a lumbar spinal drain is a useful adjunct in patients at increased risk of a cerebrospinal fluid leak. Vascularized flaps may be necessary in some patients receiving radiation therapy. Continued advances in surgical technology and the introduction of new biomaterials will facilitate the reconstruction of skull base defects following endonasal brain surgery.
Keywords: Skull base reconstruction, endonasal skull base surgery, cerebrospinal fluid leak, mucosal flap, dural reconstruction
One of the remaining challenges of endonasal skull base surgery is the endoscopic repair of large defects of the ventral skull base. The goals of reconstruction are the same as for open cranial base surgery: to provide separation of the cranial cavity and sinonasal cavity to prevent cerebrospinal fluid (CSF) leakage, pneumocephalus, and intracranial infection. In most cases, these goals can be accomplished using entirely endoscopic techniques.
Endoscopic experience with the repair of small cranial base defects following trauma, sinus surgery, and spontaneous CSF leaks (meningoencephaloceles) has demonstrated a high rate of successful repair using a variety of techniques.1 Success does not appear to be dependent on the type of reconstructive material, repair technique, or use of lumbar spinal drainage.2 A special population of patients that appear to be at increased risk of recurrent CSF leak are those presenting with spontaneous CSF leaks.3 These patients are characteristically obese, middle-aged females and measurement of CSF pressures following repair often confirms occult hydrocephalus. Despite initial success, such patients remain at risk for recurrent CSF leaks months to years following repair.
The expanded endonasal approach provides access to the entire ventral skull base from the frontal sinus to the second cervical vertebra in the sagittal plane and from the sella to the jugular foramen in the coronal plane.4,5,6 Endoscopic resection of large cranial base tumors results in larger defects than have been managed endoscopically before now. An endoscopic craniofacial resection for esthesioneuroblastoma, for example, may include the entire anterior cranial base from the frontal bone to the planum sphenoidale and to the medial walls of both orbits (~3 × 5 cm). Reconstruction of cranial base defects with the expanded endonasal approach is challenging not only because of the size of the defects but also because of the lack of supporting structures and the effects of gravity, use of nonvascularized tissues, high flow of CSF, and the risk of postoperative hydrocephalus.
This article presents our current method of reconstruction for large dural defects of the ventral skull base. Our technique reflects our experience with over 450 endoscopic skull base procedures over the past 8 years and has evolved to meet new reconstructive challenges and deal with postoperative CSF leaks.7 The majority of these surgeries were for extrasellar tumors of the ventral skull base. Reconstruction following endoscopic craniofacial resection of a sinonasal malignancy is used as an example.
TECHNIQUE
Endoscopic reconstruction of skull base defects is most readily accomplished by a team of surgeons: one provides an endoscopic view while the other uses two hands to operate. Two hands are needed to manipulate the graft, especially when suturing. The grafts can be manipulated into position with small Fukushima suctions, a ball probe, or the tip of a 1-mm angled Kerrison rongeur.
With the expanded endonasal approach, bone is removed beyond the margins of the tumor to allow reflection of the dura and to provide access to the margins of the tumor without hindrance of instrumentation. Mucosa is stripped from the surrounding bone to allow adherence of fascial and fat grafts. Once the tumor is resected and hemostasis is achieved (Fig. 1), the brain tissue is covered intradurally with a synthetic dural substitute (DuraGen®, Integra LifeSciences Corp., Plainsboro, NJ) (Fig. 2). We like to use DuraGen® here because it is pliable and sticks to the surface of the brain. It also expands slightly to help fill the dead space created by the tumor excision. The dural edge should overlap the DuraGen® by approximately 1 cm to prevent displacement of the graft as the brain or residual tumor descends or expands (Fig. 2). Although DuraGen® is successfully used in isolation for the repair of dural defects of the convexity of the brain, it lacks sufficient strength to provide a watertight seal in the intranasal environment and should not be used as the sole material for reconstruction. The size of the dural defect is measured with 0.5 × 0.5-inch pledgets and a fascial graft (Alloderm®, thin thickness) is cut to fit the defect. Alternatives to Alloderm® include fascia lata or cadaveric pericardium. We prefer the Alloderm® since it is available without donor site morbidity and is more pliable than pericardium (Fig. 3). The Alloderm® is sutured to the dural margins using nitinol U clips (Fig. 4). Nitinol is an alloy of nickel and titanium that is a temperature-dependent, shape-memory metal. This is technically demanding using an endoscopic needle holder. The U clip is released by pinching the sleeve at its junction with the nitinol clip and the needle with attached wire leader is removed (Fig. 4). The nitinol clip has memory that causes it to form a tight ring once it is released. The goal is not to get a watertight seal but to place enough U clips, especially anteriorly, to prevent migration of the graft. Oftentimes, there is insufficient dural edge for suturing along some edges of the defect. A second larger Alloderm® graft is placed extracranially to overlap the margins of the repair. Care is taken to smooth any folds in the graft and to express any fluid collections from the center of the graft. Transmitted pulsations from the brain should be evident. Fat grafts are then harvested from the abdomen using a periumbilical incision. The fat grafts are stacked on top of each other starting in the sphenoid sinus and progressing superiorly and anteriorly (Fig. 5). The surface of the fat graft is then lined with small strips of cellulose material (Surgicel, Ethicon, Inc; Somerville, NJ) to form an adherent crust. A Foley catheter (12 to 14 French) is passed transnasally from one nasal cavity across the posterior edge of the resected nasal septum to the contralateral nasopharynx, so that the tip of the catheter is not protruding into the fat grafts (Fig. 6). The balloon is slowly inflated with saline until the balloon is secure but remains slightly mobile. The pressure exerted on the fat grafts should not be excessive and should not displace the fatty tissue. Special care should be exercised if there is risk of compression of the optic nerves. The external end of the Foley catheter is secured to the skin of the cheek with tape.
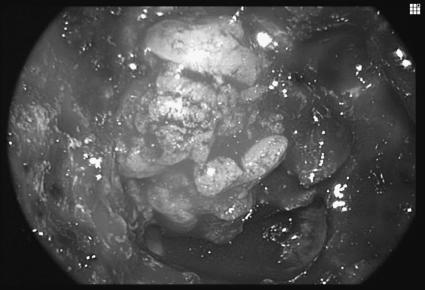
Figure 1.
Dural defect following endoscopic craniofacial resection of a neuroendocrine carcinoma of the anterior cranial base.
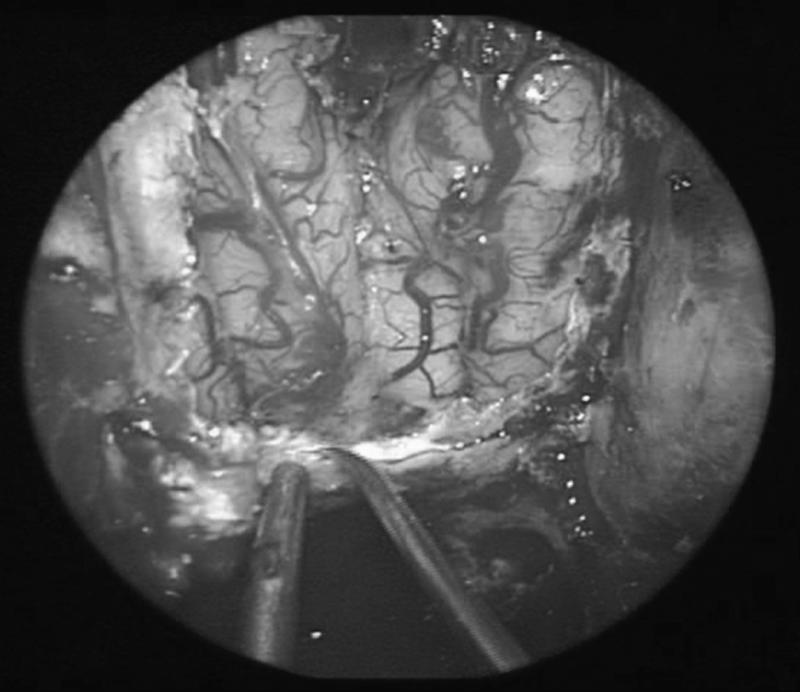
Figure 2.
A synthetic dural substitute (DuraGen®) is placed intradurally over the surface of the exposed brain so that the dural edges overlap the inlay graft.
Figure 3.
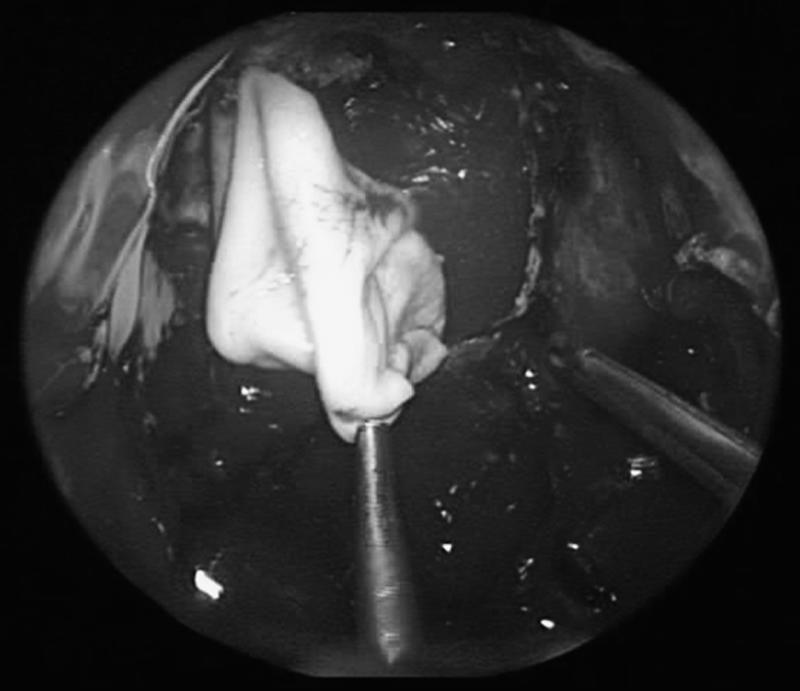
The first fascial graft (Alloderm®) is sutured to the dural margins.
Figure 4.
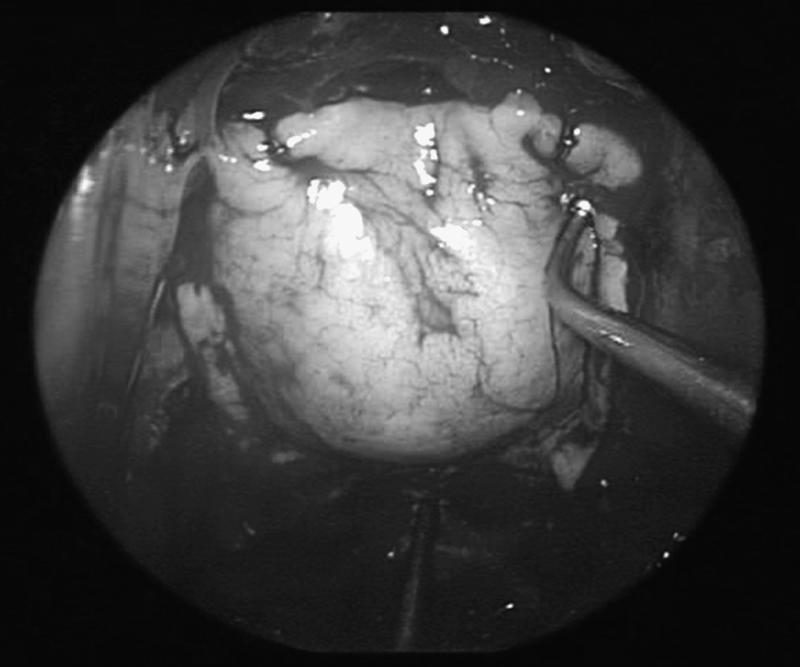
Nitinol U-clips are used to anchor the fascial graft to prevent migration or displacement by CSF.
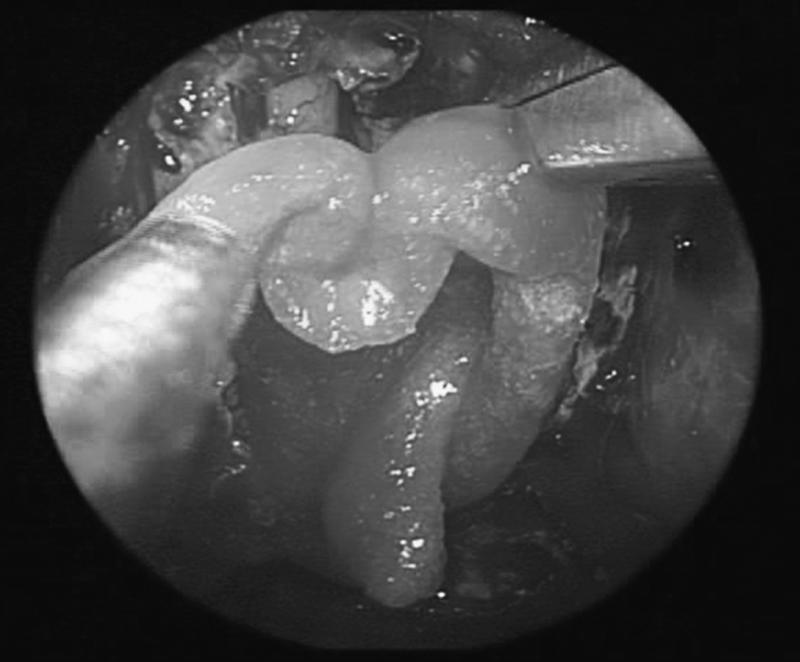
Figure 5.
Fat grafts cover the fascia and are in contact with the surrounding bone.
Figure 6.
A Foley balloon catheter crosses the nasopharynx posterior to the nasal septum, prior to inflation with saline. The surface of the fat graft is covered with Surgicel to form an adherent crust.
A postoperative CT or MRI is obtained to look for evidence of intracranial hemorrhage or pneumocephalus and to assess the position of the fat grafts and balloon catheter (Fig. 7). The balloon catheter remains in place for 4 to 7 days depending on the difficulty of the reconstruction and the risk of a CSF leak. Occasionally, the balloon ruptures prematurely due to contact with sharp bone edges, and the patient may complain of a sudden gush of fluid. No attempt is made to replace the catheter in such situations. Once the catheter is removed, nasal endoscopy can be performed to assess the adequacy of the repair and to look for evidence of a CSF leak. Pulsations of the fat graft persist for approximately 2 weeks. No manipulation of the fat grafts is performed for 4 to 6 weeks to prevent disruption of the repair. The fat graft serves as a biological dressing initially but also become partially vascularized. The nonviable surface of the fat graft forms an adherent eschar and eventually separates. Within 2 months, the surface of the fat graft becomes mucosalized and the size of the defect contracts (Fig. 8). We have not observed the need to provide rigid reconstruction of the bone to prevent herniation of the brain.
Figure 7.
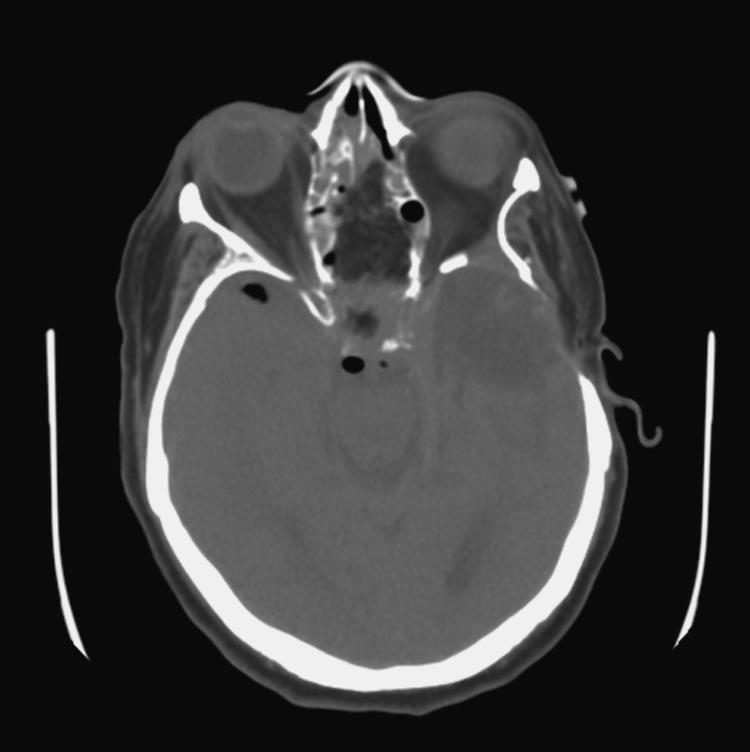
Axial CT immediately following endoscopic craniofacial resection of a meningioma demonstrates minimal pneumocephalus and fat grafts covering the skull base defect.
Figure 8.

Endoscopic view of the left nasal cavity several months following an endoscopic transplanum approach for excision of a meningioma. Complete mucosalization with viable fat graft is evident.
DISCUSSION
Small cranial base defects are successfully repaired using a variety of techniques.1,2 The defects that result with the expanded endonasal approach present several challenges that increase the risk of a postoperative CSF leak. Although the success rate is about 95% for CSF leaks associated with trauma, we have observed an increased risk of approximately 20 to 30% for patients with extrasellar tumors of the ventral skull base. Tumors such as craniopharyngiomas have an even higher risk which may be attributable to multiple factors unique to these tumors (high CSF flow, high protein content of cysts).
Factors that may increase the risk of a postoperative CSF leak in patients undergoing endoscopic surgery of the ventral skull base include a history of prior surgery, prior irradiation, a large dural defect, opening of the cisterns or communication with the ventricles, large resection volume, incomplete tumor resection, obesity, and noncompliance with postoperative instructions. Over the last 8 years, our reconstructive techniques have evolved to deal with some of these issues. Multiple fascial layers (inlay and onlay) are used to reconstruct the dura. Whenever possible, the fascial graft is sutured to the margins of the dura to prevent migration of the graft or displacement due to intracranial pressure. Fat grafts are used as a biological dressing and to promote early vascularization. Nasal packing or a balloon catheter is used to provide counterpressure extracranially. We believe that most CSF leaks start in the early postoperative period due to fluctuations in CSF pressure. This may be due to coughing or gagging during emergence from anesthesia, fluctuations in airway pressures due to obstructive sleep apnea, the effects of morbid obesity on CSF pressure, or noncompliance with activities such as nose-blowing. If there is early separation of the reconstructive tissues, a CSF tract is likely to persist. Although the routine use of lumbar spinal drains is not supported by the literature, we employ them in situations where an increased risk of CSF leak is anticipated (high flow of CSF, prior failure of repair, or conditions that predispose to postoperative hydrocephalus).
When a CSF leak develops postoperatively, there is usually separation of the fascial grafts from the dural edge focally and not a failure of the entire graft. In such situations, the defect can be repaired endoscopically with focal augmentation with another fascial graft and additional fat. In all cases, we have successfully repaired postoperative leaks endoscopically except in two patients with a history of prior irradiation.
The specialty of endoneurosurgery is rapidly evolving and advances in surgical technology and the development of new biomaterials for dural reconstruction will undoubtedly solve the problem of cranial base reconstruction. Some populations of patients may require reconstruction with vascularized tissue flaps due to the volume of the defect or the lack of vascularized tissue (prior irradiation). Options include a pedicled pericranial scalp flap, temporoparietal fascial flap, or microvascular free flap. We have observed delayed necrosis of a completely healed dural repair in one patient who received postoperative proton beam therapy. With the increased use of proton beam therapy, different reconstructive options may need to be considered in some patients.
CONCLUSION
Defects of the cranial base following endonasal brain surgery can be successfully repaired using a layered repair of inlay and onlay fascial grafts, fat grafts, and supportive packing. A postoperative CSF leak can be anticipated in about 20% of cases but is successfully dealt with endoscopically. The reconstruction of skull base defects is in constant evolution and in the absence of randomized clinical trials is often a reflection of recent successful experience. Undoubtedly, reconstructive techniques will continue to evolve as new technologies emerge and new biomaterials are introduced. Endoscopic reconstruction of large dural defects should not be an impediment to endonasal brain surgery.
DISCLOSURE
The authors have no financial interests nor receive compensation for any of the products or companies mentioned in this article.
REFERENCES
- Hegazy H M, Carrau R L, Snyderman C H, Kassam A, Zweig J. Transnasal endoscopic repair of cerebrospinal fluid rhinorrhea: a meta-analysis. Laryngoscope. 2000;110:1166–1172. doi: 10.1097/00005537-200007000-00019. [DOI] [PubMed] [Google Scholar]
- Zweig J L, Carrau R L, Celin S E, et al. Endoscopic repair of CSF leaks to the sinonasal tract: predictors of success. Otolaryngol Head Neck Surg. 2000;123:195–201. doi: 10.1067/mhn.2000.107452. [DOI] [PubMed] [Google Scholar]
- Carrau R L, Snyderman C H, Kassam A B. The management of CSF leaks in patients at risk for high-pressure hydrocephalus. Laryngoscope. 2005;115:205–212. doi: 10.1097/01.mlg.0000154719.62668.70. [DOI] [PubMed] [Google Scholar]
- Kassam A, Snyderman C H, Mintz A, Gardner P, Carrau R L. Expanded endonasal approach: the rostrocaudal axis. Part I Crista galli to the sella turcica. Available at: http://www.aans.org/education/journal/neurosurgical/July05/19-1-3.pdf. Neurosurg Focus. 2005;19:E3.. [PubMed] [Google Scholar]
- Kassam A, Snyderman C H, Mintz A, Gardner P, Carrau R L. Expanded endonasal approach: the rostrocaudal axis. Part II. Posterior clinoids to the foramen magnum. Available at: http://www.aans.org/education/journal/neurosurgical/July05/19-1-4.pdf. Neurosurg Focus. 2005;19:E4.. [PubMed] [Google Scholar]
- Kassam A B, Gardner P, Snyderman C H, Mintz A, Carrau R. Expanded endonasal approach: fully endoscopic, completely transnasal approach to the middle third of the clivus, petrous bone, middle cranial fossa, and infratemporal fossa. Available at: http://www.aans.org/education/journal/neurosurgical/July05/19-1-6.pdf. Neurosurg Focus. 2005;19:E6.. [PubMed] [Google Scholar]
- Kassam A, Carrau R L, Snyderman C H, Gardner P, Mintz A. Evolution of reconstructive techniques following endoscopic expanded endonasal approaches. Available at: http://www.aans.org/education/journal/neurosurgical/July05/19-1-8.pdf. Neurosurg Focus. 2005;19:E8.. [PubMed] [Google Scholar]