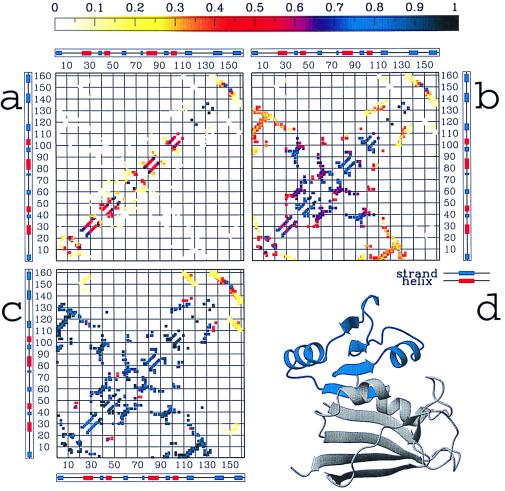
Figure 2.
The probability Qij(Q) of the native DHFR contacts being formed, resulting from the simulations at different stages of the folding process: (a) at an early stage (Q = 0.1 ± 0.05), (b) at the main intermediate, located in the interval Q = 0.4 ± 0.05 (see Fig. 1 a and c), and (c) at a late stage of the folding process (Q = 0.7 ± 0.05). In a topologically and energetically perfectly smooth funnel-like energy landscape, at any value Q during the folding, any contact (i, j) should have a probability Qij(Q) of being formed equal to Q (2). By computing Qij(Q) for each contact over different windows of the reaction coordinate Q, we can quantify the deviations from this smooth-funnel behavior and locate the early and late contacts along the folding process. It is worth noting that any deviation from “perfectly smooth” behavior is caused mainly by topological constraints, because energetic frustration has been mostly removed from the system. Different colors in the contact maps indicate different probability values from 0 to 1, as quantified by the color scale at the top. The preference for forming more local than nonlocal structure in the almost unfolded state (b) is because conformational entropy loss is smaller by forming local contacts than by pinching off longer loops (32). The most interesting result is that domain 1, identified by interactions among strands 2–5 and helices 2–3, is substantially formed at the intermediate IHF (probabilities for individual contacts grater than 0.7), whereas the formation of domain 2 (i.e., interactions among strand 1, strands 6–8, helix 1, and helix 4) is highly unfolded (contact probabilities between 0 and 0.4). Helix 1 and helix 4 are largely formed, but their interactions with the remainder of the proteins are loose (probabilities less than 0.4). Overall, this description of the structure of the main intermediate IHF—domain 1 almost formed and domain 2 largely unformed—is in agreement with the structure of IHF experimentally observed. Moreover, the latest event in the folding process (c) appears to be the formation of interactions between strands 7–8 and the remainder of the protein. This again has been experimentally determined. d illustrates the regions of the native structure that both simulations and experiments agree to indicate as formed at the intermediate IHF.