Figure 4.
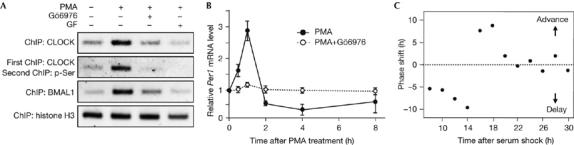
Involvement of protein kinase C-mediated CLOCK phosphorylation in the rapid activation of Per1 and phase resetting of the molecular clock. (A) Chromatin was extracted from NIH3T3 cells that were treated with 1 μM PMA for 1 h in the presence and absence of Gö6976 or GF109203X (GF). ChIP assays were carried out with specific antibodies for the indicated proteins. Re-ChIP experiments were carried out with a two-step immunoprecipitation with CLOCK and phospho-Ser antibodies (pSer). The association of each protein with the Per1 promoter was analysed by PCR using a primer set flanking the proximal E-box. ChIP data of histone H3 are shown as a control. (B) Cells were treated with 1 μM PMA for 2 h, and the medium was replaced with serum-free medium. A set of cells was incubated with Gö6976 for 1 h before treatment with PMA. Per1 messenger RNA levels were determined by reverse transcription–PCR and normalized with those of G3PDH. Data are shown as means±s.e.m. of three independent experiments. (C) Phase responses to a 15 min PMA pulse. NIH3T3 cells stably transfected with a plasmid containing the Per1-dsLuc were synchronized by serum shock and then treated with PMA for 15 min at various time points. The Per1-dsLuc activities were monitored continuously using a real-time measuring system and the resulting shift in the second peak time of the bioluminescence was expressed as phase shift of the molecular clock. BMAL1, brain and muscle Arnt-like protein 1; ChIP, chromatin immunoprecipitation; PMA, phorbol myristic acetate.
