Figure 2.
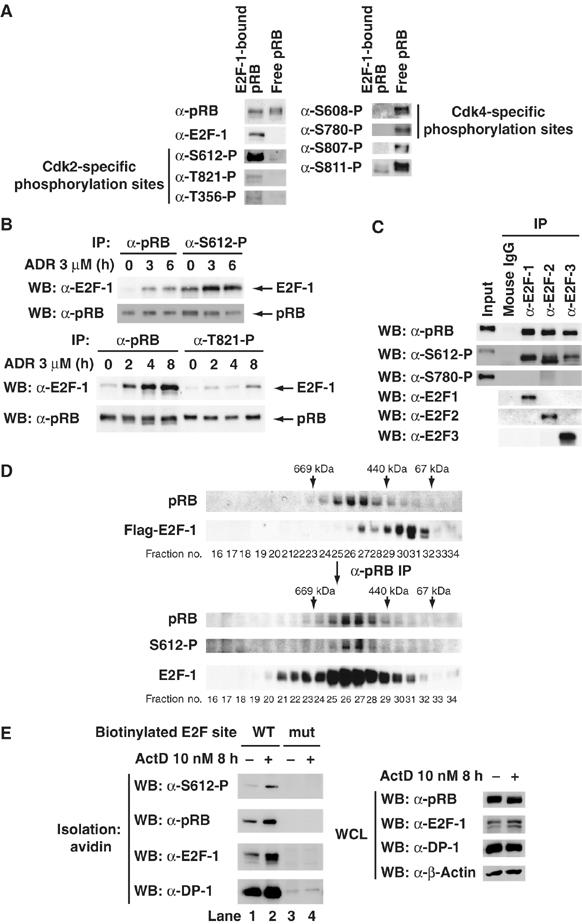
pRB phosphorylated at Ser612 prefers to bind to E2F-1. (A) Analysis of phosphorylation profiles in E2F-1-bound pRB and E2F-1 unbound pRB after DNA damage. MCF7 cells were treated with 3 μM adriamycin (ADR) for 24 h. Cell lysates were subjected to two rounds of immunoprecipitation with anti-E2F-1 antibody (E2F-1-bound pRB). Next, the residual supernatants were subjected to immunoprecipitation with anti-pRB antibody (free pRB). Immunoprecipitates normalized for the amount of precipitated pRB were subjected to a Western blot analysis with the indicated antibodies. (B) E2F-1 is effectively co-precipitated with Ser612- but not Thr821-phosphorylated pRB. MCF7 cells were treated with 3 μM ADR for the indicated periods. Cell lysates were subjected to immunoprecipitation with the indicated antibodies. Immunoprecipitates normalized for the amount of precipitated pRB were subjected to a Western blot analysis with the indicated antibodies. (C) E2F-2 and E2F-3 effectively bind to Ser612-phosphorylated pRB as well as E2F-1 does. Lysates from MOLT-4 cells were subjecied to immunoprecipitation with the indicated antibodies. Immunoprecipitates normalized for the amount of precipitated pRB were subjected to a Western blot analysis with the indicated antibodies. (D) Ser612-phosphorylated pRB forms a complex with E2F-1 in vivo. MCF7 cells were transiently transfected with Flag–E2F-1. Cells were treated with 3 μM ADR for 24 h. Cell lysate was immunoprecipitated with anti-Flag antibody-conjugated beads. Flag–E2F-1 complex was eluted from the beads with Flag peptide and further purified by gel filtration on a column of superose 6. Upper panels are an immunoblot analysis of gel filtration column fractions using antibodies. pRB in the E2F-1 complex was concentrated using anti-pRB antibody to immunoprecipitate pRB from fractions of a superose 6 gel filtration column, and Ser612-phosphorylated pRB in the E2F-1 complex was detected (lower panels). (E) Ser612-phosphorylated pRB can be recruited to E2F binding sites through its interaction with the E2F-1/DP-1 heterodimer. MCF7 cells were exposed to 10 nM ActD for 8 h, and then cell extracts were subjected to a pull-down analysis using biotinylated oligonucleotides harboring either a wild-type E2F site or a mutated site. Bound proteins were then analyzed using the indicated antibodies.
