Figure 6.
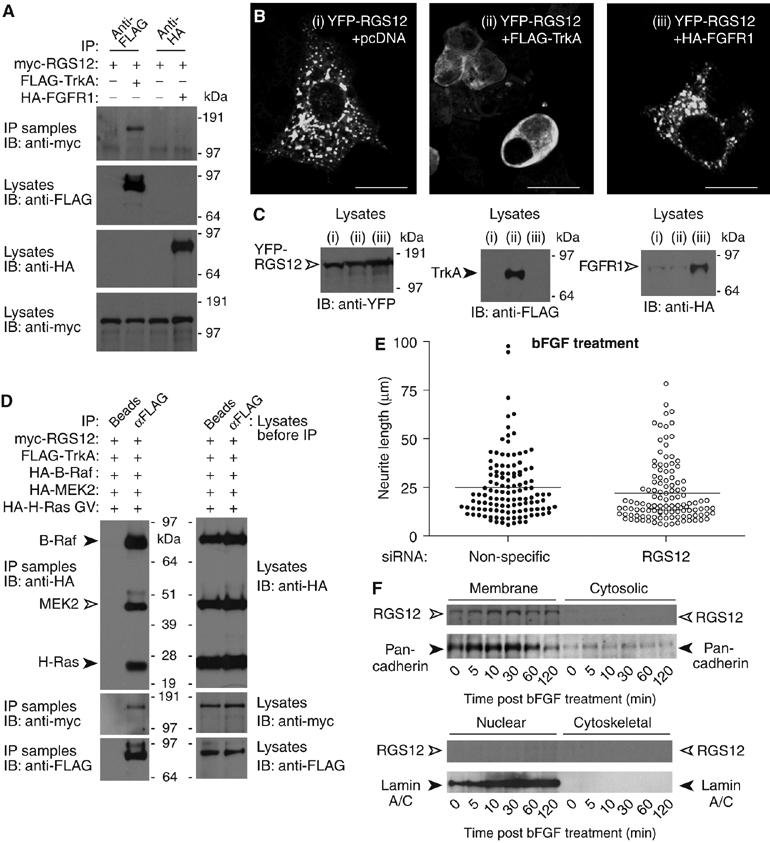
RGS12 forms a multiprotein complex containing TrkA and shows selectivity for TrkA signaling versus FGFR signaling. (A) HEK 293T cells were cotransfected with myc-RGS12, and either empty vector, FLAG-TrkA, or HA-FGFR1 plasmids. Anti-FLAG or anti-HA antibody was used to immunoprecipitate FLAG-TrkA or HA-FGFR1, respectively. Anti-myc was used to detect associated myc-RGS12 protein. (B) HEK 293T cells were cotransfected with YFP-RGS12 and either (i) pcDNA3.1 empty vector, (ii) FLAG-TrkA, or (iii) HA-FGFR1 vectors and imaged by confocal microscopy; scale bar represents 20 μm. (C) HEK 293T cells were transfected as described in panel B. At 72 h post-transfection, cells were lysed and immunoblotted to verify expression of YFP-RGS12, FLAG-TrkA, and HA-FGFR1. (D) HEK 293T cells were transfected with myc-RGS12, FLAG-TrkA, HA-B-Raf, HA-MEK2, and HA-H-Ras G12V plasmids. Lysates were immunoprecipitated with beads alone or anti-FLAG antibody. Anti-HA and anti-myc were used to detect associated myc-RGS12, HA-B-Raf, HA-MEK2, and HA-H-Ras G12V. (E) PC12 cells were transfected with non-specific or RGS12 siRNA for 24 h and treated with 100 ng/ml bFGF for an additional 48 h. Differences in neurite length were quantitated as described in Figure 4C. Median neurite lengths of non-specific siRNA-transfected cells were not significantly different from those of RGS12 siRNA-transfected cells (P>0.03; Mann–Whitney test). (F) PC12 cells were stimulated with bFGF for the indicated durations. Cells were then fractionated into membrane, cytosolic, nuclear, and cytoskeletal fractions as in Figure 5F. Proteins resolved by SDS–PAGE were immunoblotted with anti-RGS12, or antibodies against nuclear envelope marker lamin A/C or plasma membrane marker anti-pan-cadherin.
