Figure 4.
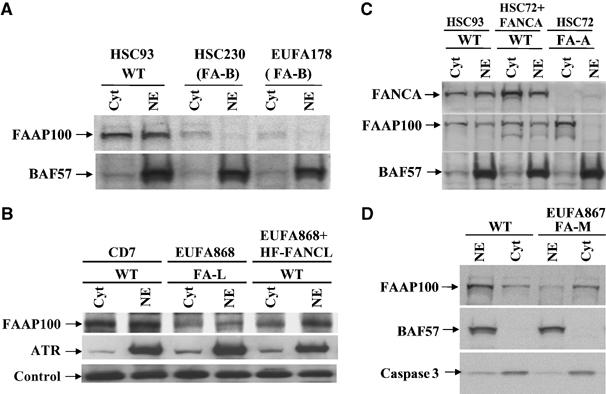
FAAP100 is unstable in FA patient cells deficient of FANCB or FANCL, and its nuclear localization is defective in cells deficient of FANCA or FANCM. (A) Immunoblotting shows that the FAAP100 protein level is reduced in nuclear (NE) and cytoplasmic (cyt) extracts of two different FA patient cell lines deficient of FANCB (FA-B). A cell line from a normal individual, HSC93, was included as a wild-type control (WT). BAF57, a component of SWI/SNF chromatin remodeling complex, was used as a loading control. (B) Immunoblotting shows that the FAAP100 protein level is reduced in the FA patient cell line deficient of FANCL (FA-L). Its level is increased when the same cell line was complemented by reintroduction of HF-FANCL (‘WT') (Supplementary Figure 1). ATR was used as a control for nuclear extract and a crossreactive band was used as a loading control. (C) Immunoblotting shows that the level of FAAP100 is strongly reduced in the nuclear extract of a FA patient cell line (HSC72) deficient of FANCA (FA-A). Importantly, in the same cell line complemented with expression of exogenous FANCA (HSC72+FANCA), the level of FAAP100 in nuclear extract becomes normal. (D) Immunoblotting reveals that the level of FAAP100 is reduced in the nuclear extract of a FA patient cell line (EUFA867) deficient of FANCM. BAF57 and caspase 3 are loading controls for nuclear and cytosolic extracts, respectively.
