Figure 3a.
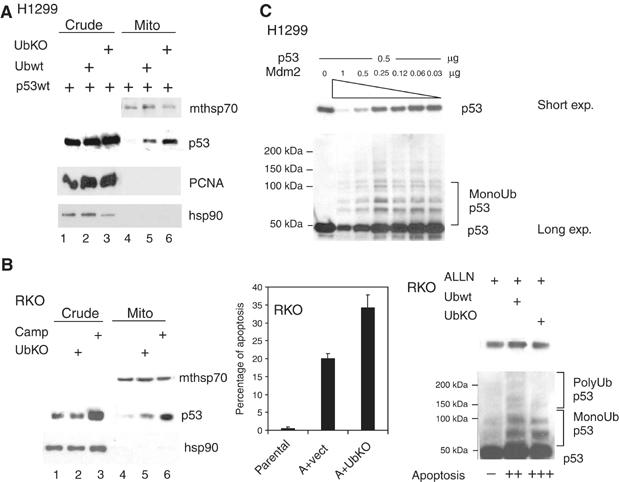
Mdm2-mediated monoubiquitylation promotes mitochondrial p53 translocation and the mitochondrial p53 apoptotic program. (A) Immunoblot of H1299 cells cotransfected with wtp53 and equal amounts of either Ubwt or UbKO. Mitochondrial loading was titrated down to the point where p53wt alone did not produce detectable translocation (lane 4) in order to show increased translocation in lanes 5, 6. (B, left) Immunoblot of RKO cells mock- or UbKO transfected. Camp-treated cells were used as control. (B, middle) RKO cells were transfected, as indicated, or left untreated. Twenty-four hours later α-amanitin was added to the culture medium where, indicated, for an additional 14 h to block transcription. Apoptosis was determined by TUNEL. (B, right) Increased apoptosis in RKO cells is accompanied by enhanced p53 monoubiquitylation after expression of Ubwt or UbKO. RKO cells were treated, as indicated, and normalized amounts of p53 were immunoblotted. (C) The fate of p53 depends on the p53:Mdm2 ratio in the cell. p53-null H1299 cells were cotransfected with 0.5 μg of p53 plasmid and decreasing concentrations of the Mdm2 plasmid. Note that whereas high concentrations of Mdm2 cause polyubiquitylation and degradation of p53, low concentrations of Mdm2 preferentially catalyze monoubiquitylation and stabilization of p53.
