Figure 3b.
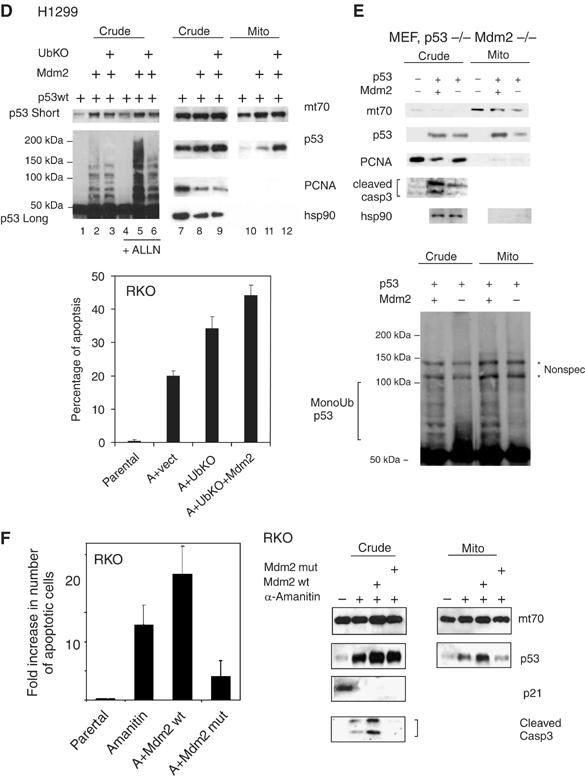
(D) Mdm2-mediated monoubiquitylation of p53 promotes its mitochondrial targeting. Right: immunoblot of crude lysates and purified mitochondria of H1299 cells transfected with wtp53, Mdm2wt and UbKO, left: short and long DO-1 exposures of the same crude lysates +/− proteosome inhibitor ALLN to show preferential Mdm2-mediated p53 monoubiquitylation upon UbKO expression. Sample loading was normalized for the equal amount of nonubiquitilated p53. Lower panel: RKO cells were transfected, as indicated, or left untreated. Twenty-four hours later, α-amanitin was added to the medium, where indicated, for an additional 14 h to block transcription. Apoptosis was determined by TUNEL. (E) Mdm2 deficiency causes defects in mitochondrial p53 translocation and caspase 3 activation. Early passage p53−/− Mdm2−/− double knockout MEFs were retrovirally transduced with p53wt and Mdm2wt as indicated. After 36 h, crude lysates and purified mitochondria were immunoblotted as indicated, including an antibody that is specific for the activated form of caspase 3. Mitochondrial loading was normalized for equal total cellular p53 protein. Lower panel: Mdm2-mediated enhanced mitochondrial p53 translocation is associated with enhanced p53 monoubiquitylation. p53 immunoblot after long exposure. * represents nonspecific bands. (F) α-Amanitin stabilizes wtp53, induces its mitochondrial translocation, activates caspase 3 and induces apoptosis in the absence of transcription, as indicated by, for example, the p53 target gene p21. Expression of wtMdm2 enhances all of these effects, which translates into enhanced apoptosis via the mitochondrial p53 program. In contrast, expression of the catalytically inactive dominant-negative Ring-finger mutant of Mdm2 (C438L) suppresses all of these effects below the level of endogenous Mdm2 (seen with α-amanitin alone). RKO cells were transfected as indicated and 20 h later cells were treated with α-amanitin for an additional 14 h, except for parental cells. Crude and mitochondrial extracts were immunoblotted as indicated. Mitochondrial loading was normalized for equal mitochondrial protein. Apoptosis was assayed by caspase 3 cleavage and bottom by TUNEL staining. Error bars in (C), (E) and (G) are s.d. from three independent experiments.
