Figure 3.
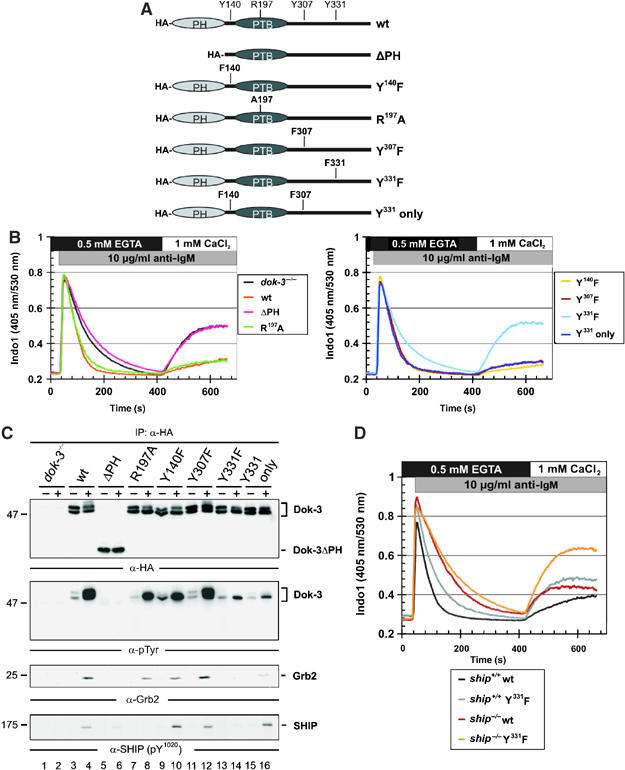
Dok-3 and Grb2 build a functional unit, that inhibits Ca2+ flux independent of SHIP. (A) Schematic representation of expression constructs encoding HA-tagged versions of wild-type Dok-3, a PH domain deletion mutant (ΔPH) or mutants encompassing amino-acid exchanges depicted in single-letter code. (B) Expression vectors were introduced by retroviral transduction in dok-3−/− mutants and BCR-induced Ca2+ mobilization of the transfectants was measured by flow cytometry, as described in the legend to Figure 2. Wild-type DT40 cells and empty vector transfectants of dok-3−/− mutants served as control (see inlay for color code). (C) Wild-type and DT40 variants described in (B) were left untreated (−) or BCR-activated (+) and lysates were subjected to anti-HA immunoprecipitation. Expression and tyrosine phosphorylation of Dok-3 proteins, as well as their association to Grb2 and SHIP, were detected by sequential immunoblotting with antibodies to HA, pTyr, Grb2 and SHIP (upper to lower panels, respectively). (D) BCR-induced Ca2+ fluxes were analyzed as described in the legend to Figure 2 in SHIP-deficient DT40 cells (ship−/−, brown) and ship−/− transfectants expressing a Dok-3 Y331F variant that counteracts Ca2+ inhibition by endogenous wild-type Dok-3 (orange). As control, parental DT40 cells, which are positive for endogenous SHIP and Dok-3 (black), and the Dok-3 Y331F transfectants (gray) were analyzed in parallel, demonstrating the dominant-negative function of Dok-3 Y331F.
