Figure 4.
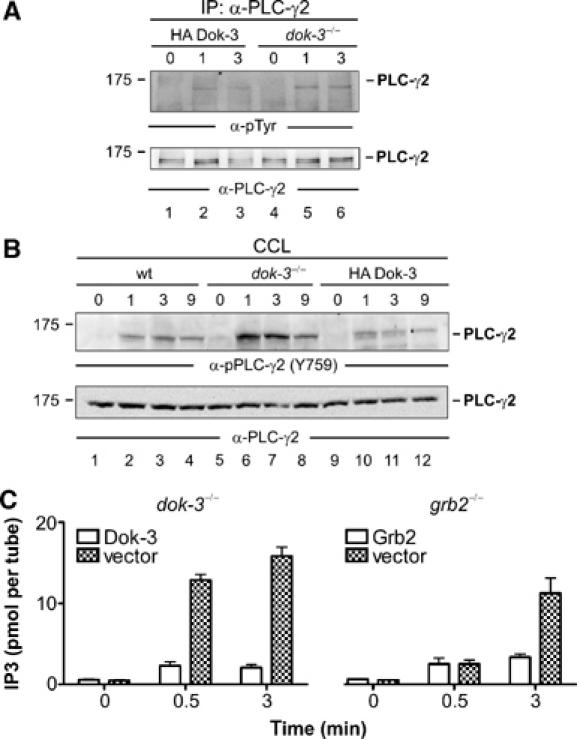
The Dok-3/Grb2 module attenuates PLC-γ2 activity. (A) Dok-3-deficient DT40 mutants (lanes 4–6) and reconstituted cells expressing HA-tagged wild-type Dok-3 (lanes 1–3) were left untreated (0) or stimulated through their BCRs for the indicated times (min). Lysates were subjected to anti-PLC-γ2 immunopurification and proteins obtained were analyzed by anti-pTyr and anti-PLC-γ2 immunoblotting (upper and lower panels, respectively). (B) Parental DT40 cells (lanes 1–4), dok-3−/− mutants (lanes 5–8) and HA-Dok-3-reconstituted transfectants (lanes 9–12) were left untreated (0) or stimulated through their BCRs for the indicated times (min). Cleared cellular lysates (CCL) were subjected to immunoblot analysis with antibodies that specifically detect PLC-γ2 phosphorylation at the Btk-dependent phospho-acceptor site corresponding to Y759 in human PLC-γ2 (upper panel). Equal protein loading was confirmed by reprobing the membrane with anti-PLC-γ2 antibodies (lower panel). Relative molecular mass of marker protein is indicated in (A) and (B) on the left in kDa. (C) DT40 mutant cells deficient for either Dok-3 (left panel) or Grb2 (right panel) and the empty vector control transfectants (open and filled bars, respectively) were left untreated (0) or BCR-activated for 0.5 or 3 min. IP3 levels in these cells were measured using a competitive binding assay with radiolabelled IP3-binding proteins. Error bars represent s.e.m. of three independent experiments with double preparation.
