Figure 2.
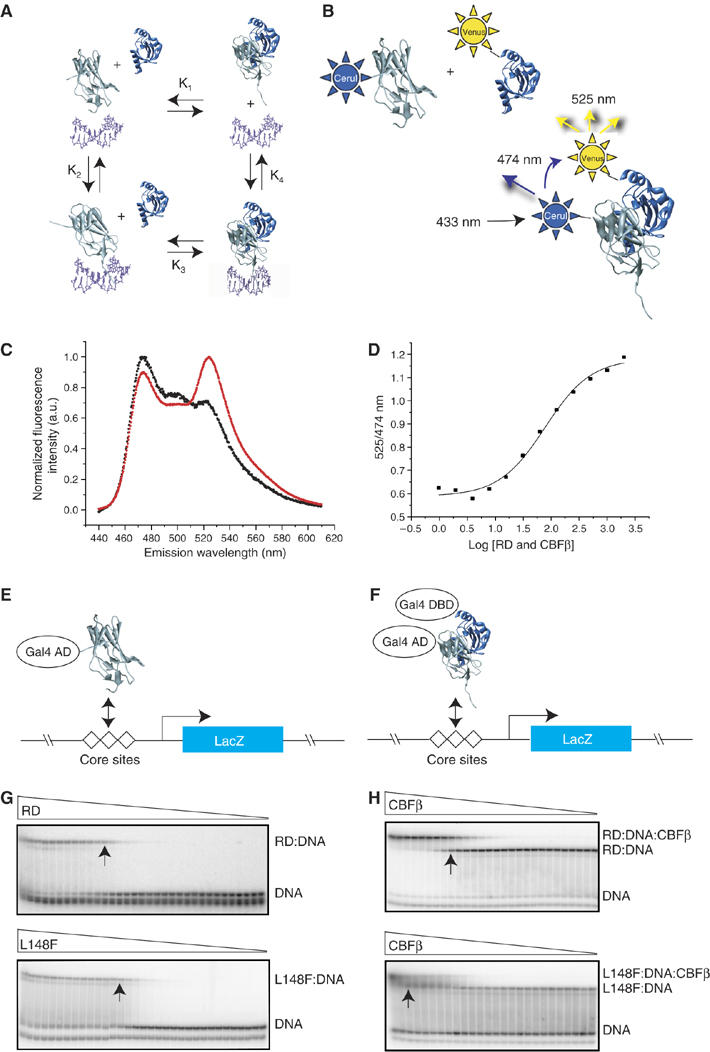
DNA and CBFβ binding by mutant RDs. (A) Schematic diagram of the potential interactions between the RD (gray), CBFβ (blue), and DNA (purple). These interactions can be described by four equilibrium constants: K1, the dissociation constant for the RD-CBFβ heterodimer in the absence of DNA; K2, the dissociation constant for the RD–DNA complex; K3, the dissociation constant for CBFβ binding to the RD–DNA complex; and K4 which describes binding of the RD–CBFβ heterodimer to DNA. (B) FRET assay to determine K1. Cerulean (Cer), an optimized version of the cyan fluorescent protein (Rizzo et al, 2004), was fused to the N-terminus of the RD, and the YFP derivative Venus (Rizzo et al, 2004) was fused to the N-terminus of CBFβ. Cerulean was excited at 433 nm and emission from Cerulean and Venus detected at 474 and 525 nm, respectively. (C) Fluorescence spectra of Cerulean-RD and Venus-CBFβ showing the FRET effect. The black curve is the spectrum of Cerulean-RD+Venus-CBFβ at a concentration of 25 nM (4.5-fold below K1). The red curve is the spectrum of Cerulean-RD+Venus-CBFβ at a concentration of 400 nM (3.6-fold above K1). (D) FRET assay of the WT RD binding to CBFβ. The samples were excited at 433 nm, and the ratio of Venus-CBFβ/Cerulean-RD emission peaks (525/474 nm) was plotted at different protein concentrations to generate a binding curve. (E) Yeast one-hybrid assay for RD binding to three core sites driving lacZ expression. Mutations that increase K2 by ⩾10-fold decrease β-galactosidase activity in a yeast one-hybrid filter assay to undetectable levels (Li et al, 2003). Visible but decreased β-galactosidase activity in the filter assay reflects K2 increases in the 3–10-fold range. (F) Modified yeast one-hybrid assay to measure binding of the RD:CBFβ heterodimer to DNA. Although the Gal4 DNA-binding domain is fused to CBFβ in the modified yeast one-hybrid assay, there are no Gal4-binding sites on the promoter driving lacZ and therefore CBFβ's activity is mediated only through the core sites. CBFβ increases the affinity of the RD for DNA by approximately 10-fold. RD mutants that can bind CBFβ and have K2 increases in the 10–90-fold range and correspondingly no β-galactosidase activity in the one-hybrid assay, yield β-galactosidase signals in the modified one-hybrid assay (Li et al, 2003). On the other hand, RD mutants with K2 values that are >100-fold higher than the WT RD produce very weak or no β-galactosidase activity in the modified yeast one-hybrid assay (Li et al, 2003). (G) EMSA measuring the affinity of the WT RD (top) and the L148F RD (bottom) for DNA (K2). Triangles indicate decreasing concentrations of RDs (WT RD, 2 × 10−6 to 4 × 10−15 M; L148F, 1 × 10−5 to 3 × 10−14 M). Arrows indicate lanes in which the RD concentration approximates K2. (H) EMSA measuring the affinity of the WT RD:DNA complex (top) and L148F RD:DNA complex (bottom) for CBFβ (K3). Triangles indicate decreasing concentrations of CBFβ (for the WT RD, 6 × 10−6–4 × 10−14 M; L148F, 2 × 10−5–4 × 10−12 M). Arrows indicate lanes in which the CBFβ concentration approximates K3.
