Figure 1.
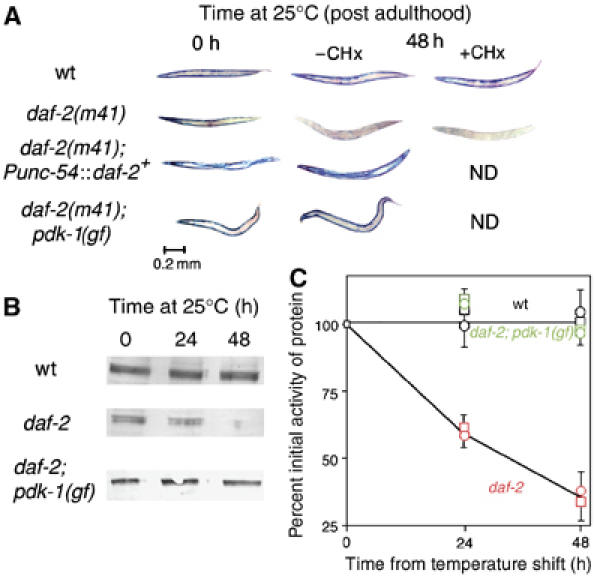
Intramuscular DAF-2 signaling opposes protein degradation in muscle. Animals were grown to mid-adulthood at 15°C and then shifted to 25°C for an additional 48 h either without or with the protein synthesis inhibitor CHx (400 μg/ml) added 6 h before temperature upshift. (A) Histochemical stain for β-galactosidase activity in muscle (blue). Reporter activity in wild-type animals (top row) remains stable after temperature shift even when treated with CHx. daf-2(m41) animals (second row) show a loss of reporter activity after temperature upshift; this is not prevented by CHx pretreatment. The loss of activity in daf-2(m41) animals is rescued by muscle-specific expression of wild-type DAF-2 (Punc-54∷daf-2+, third row) or by a gain-of-function mutation pdk-1(mg142) (bottom row). (B) Immunoblot (Zdinak et al, 1997) of 146 kDa myosin-β-galactosidase fusion protein (using monoclonal anti-β-galactosidase antibody) in 30-worm lysates confirms that the loss of staining seen in (A) corresponds to degradation of the reporter protein. (C) Quantitation (NIH Image software) of protein levels in (B) and of β-galactosidase activity by fluorimetric assay (Zdinak et al, 1997) of 10-worm lysates (means±s.d. of three independent determinations) confirms that protein degradation (squares) and loss of activity (circles) proceed at similar rates in daf-2 animals (red) whereas no degradation occurs in wild-type (black) or daf-2; pdk-1 (gf) animals (green).
