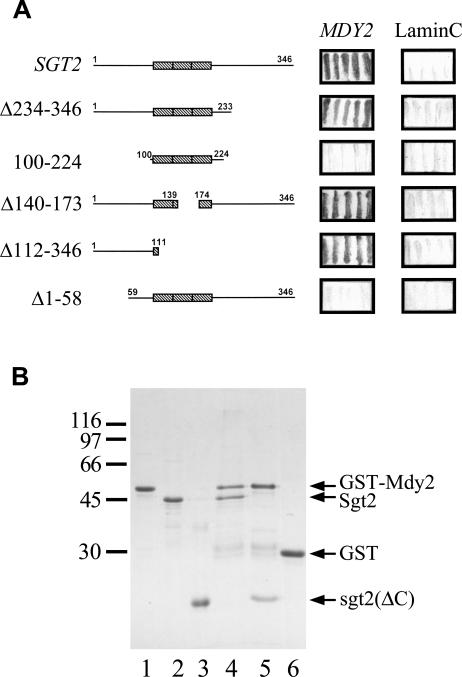
Fig 4.
The N-terminal region of Sgt2 interacts with Mdy2. (A) Yeast 2-hybrid analysis. The open reading frame of SGT2 and its deletion mutants were constructed in pAS2-1, and MDY2 was constructed in pGAD-Cx. The plasmids were transformed into yeast strain Y190 for 2-hybrid assays, and β-galactosidase activity was determined by filter assays. Lamin C in pACT2 was used as a negative control, and the resulting blue filters are shown here. (B) Glutathione S-transferase (GST) pull-down assays. GST-Mdy2 and GST were purified and allowed to bind to glutathione (GSH)-Sepharose. The resin was then mixed with purified Sgt2 or its N-terminal fragment, sgt2(ΔC). The bound proteins were eluted with 50 mM GSH and were then analyzed with sodium dodecyl sulfate gel electrophoresis. A Coomassie Brilliant Blue–stained gel is shown. Lanes 1, 2, and 3 contain purified GST-Mdy2, Sgt2, and its N-terminal fragment, respectively. Lanes 4 and 5 contain GST-Mdy2 incubated with Sgt2 and the N-terminal fragment of Sgt2, respectively. Purified GST incubated with Sgt2 (lane 6) or the N-terminal fragment of Sgt2 (not shown) were used as controls. Both Sgt2 and its N-terminal fragment failed to associate with GST. The molecular mass markers are β-galactosidase (116 000), phosphorylase (97 000), bovine serum albumin (66 000), ovalbumin (45 000), and carbonic anhydrase (30 000)