Abstract
It has long been known that cell-cell adhesiveness is generally reduced in human cancers. Tumor cells are dissociated throughout the entire tumor masses of diffuse-type cancers, whereas those of solid tumors with high metastatic potentials are often focally dissociated or dedifferentiated at the invading fronts. Thus, both irreversible and reversible mechanisms for inactivating the cell adhesion system appear to exist. This paper focuses on the cadherin system, which mediates Ca2+-dependent homophilic cell-cell adhesion. The E (epithelial)-cadherin-mediated cell adhesion system in cancer cells is inactivated by multiple mechanisms corresponding to the pathological features described above. Mutations have been found in the genes for E-cadherin and its undercoat proteins, α- and β-catenins, which connect cadherins to actin filaments and establish firm cell-cell adhesion. Transcriptional inactivation of E-cadherin expression was shown to occur frequently in tumor progression. E-cadherin expression in human cancer cells is regulated by CpG methylation around the promoter region. The cadherin system interacts directly with products of oncogenes, eg, c-erbB-2 protein and the epidermal growth factor receptor, and of the tumor suppressor gene, adenomatous polyposis coli (APC) protein, through β-catenin, which may be important in signal transduction pathways contributing to the determination of the biological properties of human cancers. In conclusion, inactivation of the E-cadherin system by multiple mechanisms, including both genetic and epigenetic events, plays a significant role in multistage carcinogenesis.
Cell-Cell Adhesion and Human Cancers
Cell-cell adhesion participates in histogenesis and plays a critical role in the establishment and maintenance of cell polarity and cell society. It was known as early as the 1940s that the mutual adhesiveness of cancer cells is significantly weaker than that of the corresponding normal cells. 1,2 Reduced cell-cell adhesiveness allows cancer cells to disobey the social order, resulting in destruction of the histological structure, the morphological hallmark of malignant tumors. In cancers in vivo, particularly the diffuse type, tumor cells are dissociated throughout the entire tumor masses, lose their cell polarity, and infiltrate the stroma in a scattered manner. One of the most characteristic features of cultured cancer cells in vitro is loss of “contact inhibition,” which reflects disordered signal transduction from cell-cell adhesion to cell growth. Moreover, invasion and metastasis, which are the most life-threatening properties of malignant tumors, are considered to be later, but critically important, carcinogenetic steps. The invasion and metastatic processes themselves consist of sequential steps involving host-tumor interactions. In order for a metastatic nodule to form, cancer cells must leave the primary cancer nests, invade the surrounding host tissue, enter the circulation, lodge in a distant vascular bed, extravasate into the target organ, and proliferate. 3 The dissociation of cancer cells from cancer nests is a crucial step and the suppression of cell-cell adhesiveness may trigger the release of cancer cells from the primary cancer nests and confer invasive properties on a tumor. Indeed, the tumor cells of solid tumors with high metastatic potentials are often focally dissociated at the invading fronts. Therefore, reduced cell-cell adhesiveness is considered indispensable for both early and late carcinogenetic steps. Human cancers appear to possess both irreversible and reversible mechanisms for inactivating the cell-cell adhesion system.
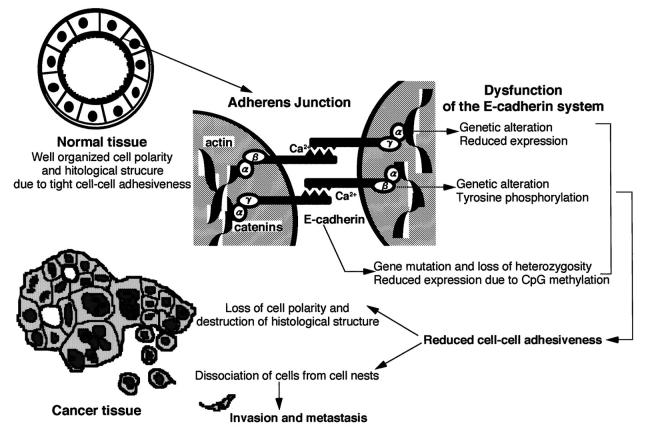
The cell-cell adherens junction is a specialized region of the plasma membrane connected with cytoskeletal actin filaments, where cadherins act as Ca2+-dependent adhesion molecules (Figure 1) ▶ . 4,5 Cadherin molecules are integral membrane glycoproteins with a single transmembrane domain. The extracellular domain of E-cadherin, the major cadherin in epithelial cells, is composed of a series of components, each comprising about 110 amino acid residues. Each of these components contains two putative Ca2+-binding motifs, which are considered to play key roles in Ca2+-protein and protein-protein interactions. 4,5 Cadherins mainly interact in a homophilic manner, eg, E-cadherin binds selectively to E-cadherin, and the amino terminal 113 amino acid residues are essential for this selective adhesiveness. 4,5 When a nonionic detergent extract of cells was immunoprecipitated with an anti-cadherin antibody, three cytoplasmic proteins, α- and β-catenins and plakoglobin (γ-catenin), were co-precipitated. Catenins interact with cadherins through the cytoplasmic domain, which exhibits the strongest degree of homology between different members of the cadherin family. Mutant cadherin molecules lacking the catenin-binding sites failed to interact with the actin filaments, indicating that interactions between cadherins and cytoskeletal proteins through catenins confer stability on the cell-cell adherens junction (Figure 1) ▶ . 4,5
Figure 1.
Multiple mechanisms of inactivation of the E-cadherin-mediated cell adhesion system in human cancers.
Because the cell-cell adherens junction is mechanically stable, it is believed to play a role in tissue morphogenesis. When E-cadherin is sufficiently active, epithelial cells, including cancer cells, cannot disrupt their mutual connections, but suppression of E-cadherin activity may trigger the release of cancer cells from primary cancer nests. In fact, in vitro experiments on cultured cells revealed that E-cadherin has invasion-suppressing properties. 6-8 We and other groups have shown that the E-cadherin-mediated cell adhesion system in human cancers is inactivated by multiple mechanisms. The mechanisms responsible for and the significance of inactivation of this system are discussed in the following sections.
Inactivation of the E-Cadherin-Mediated Cell Adhesion System as a Result of Genetic Alteration
The tumor cells in diffuse-type cancers are dissociated throughout the entire tumor masses, leading us to think that the E-cadherin-mediated cell adhesion system in cancer cells may be inactivated as a consequence of genetic alterations. We started to explore this hypothesis by looking for mutations in human cancer cell lines derived from clinical specimens of patients with diffuse-type cancers and reported genetic alterations of α-catenin in a lung cancer cell line in 1992, the first evidence that our hypothesis was correct. 9,10 Mutations of the genes for E-cadherin and catenins were detected subsequently in other human cancer cell lines. 11,12 Some studies overcame the effects of sample contamination by noncancerous cells, which is one of the major problems encountered when analyzing diffuse-type cancers in vivo, and found evidence that genetic alterations of members of the cadherin-catenin complex occurred in vivo (Figure 1) ▶ . 13-15
The reported structural abnormalities of the E-cadherin molecule include gene mutations resulting in exon skipping 13 and/or in-frame and frame-shift deletions and insertions. 11 In some cases, exon skipping and insertions were confirmed to be caused by mRNA splicing errors. 11,13,15 Some examples of point mutations and in-frame mutations in the coding sequence near the functionally important regions of E-cadherin were found and assumed to abolish the activity of this molecule. 11,14 The E-cadherin mutations reported so far occurred in 9 of the 16 exons, but they tended to accumulate in exons 6 through 10, which correspond to the extracellular domain.
Signet ring cell carcinomas of the stomach, typical of diffuse-type cancers, have been analyzed in vivo. Cellular atypia is rather mild in these carcinomas and their most obvious characteristic, which occurs even in the intramucosal lesions, is the complete loss of cell-cell adhesiveness, resulting in destruction of the histological structure. E-cadherin gene mutations in introns resulting in skipping of exon 9 were detected in the intramucosal lesions of signet ring cell carcinomas and in deeply invaded areas. 15 These findings suggest that genetic alterations of the E-cadherin gene are involved not only in the late carcinogenetic events of invasion and metastasis but also during the early developmental stages of some histological types of human cancer. The incidence of mutations resulting in skipping of exon 8 or 9 in diffuse-type stomach cancers was high, about 40%, but no mutations were detected in intestinal-type stomach cancers. 13
Cell lines possessing E-cadherin gene mutations show loss of the wild-type E-cadherin allele, suggesting that E-cadherin dysfunction in such cell lines results from a two-hit mechanism, a combination of the loss of one allele and a mutation in the remaining one, as occurs in the classical tumor suppressor genes. 11 It is noteworthy that loss of heterozygosity on the long arm of chromosome 16, to which the E-cadherin gene has been assigned, is detected frequently in metastasizing human cancers derived from the liver, prostate, and breast. In fact, genomic mutation of the E-cadherin gene accompanied by loss of heterozygosity on 16q was detected in invasive lobular carcinomas of the breast. 14 Recently, a large kindred study 16 of early-onset, diffuse-type stomach cancers from New Zealand revealed a germ-line mutation located at the same position as the somatic mutation 11 described above, indicating that the E-cadherin gene actually satisfies the criteria for tumor suppressor genes.
The reported genetic alterations of the α-catenin gene in human cancer cell lines include homozygous deletion, which may affect the RNA splicing recognition signal and result in both mRNA splicing errors and marked mRNA instability. 10,17 Human cultured cancer cells possessing genetically altered α-catenin regained their cell-cell adhesiveness when transfected with wild-type α-catenin cDNA, 18 providing the first evidence that functional α-catenin is indispensable to the E-cadherin system.
Genetic alterations of β-catenin abolishing cell-cell adhesiveness have been observed in two cell lines derived from a signet ring cell carcinoma of the stomach. Homozygous deletion of a part of the β-catenin gene, causing an identical in-frame mRNA deletion of β-catenin, was identified in both these cell lines, 12 and the truncated β-catenin lacked a region through which β-catenin is now known to interact with α-catenin. Although the truncated β-catenin was co-precipitated in immunoprecipitation experiments using an anti-human E-cadherin monoclonal antibody, α-catenin was not, indicating that the interaction between α-catenin and E-cadherin is not direct but mediated by β-catenin. These data indicated that genetic alteration of β-catenin can disrupt the interaction between E-cadherin and α-catenin and may participate in the loosely adhesive growing patterns of these cell lines. Moreover, both cell lines were established from the ascites of the same patient, suggesting that the mutation had already occurred and played a role in the invasive growth pattern of the tumor in vivo. 12
Inactivation of the E-Cadherin-Mediated Cell Adhesion System Due to Reduced Expression
In an attempt to establish the significance of reduced expression of E-cadherin in human cancer tissues in vivo, immunohistochemical examinations using an anti-human E-cadherin monoclonal antibody were performed. 19-21 E-cadherin was expressed uniformly in most cohesive stomach cancer cells, but it was not detected in some diffuse-type stomach cancer cells, which lacked tight cell-cell adhesion. 19,20 Many other studies on E-cadherin protein expression in various human cancers have been reported. Generally, E-cadherin expression was found to be strong in well differentiated cancers, which maintain their cell-cell adhesiveness and are less invasive, but reduced in undifferentiated cancers, which have lost their cell-cell adhesion and show a strong invasive tendency (reviewed in Ref. 22 ). Therefore, inactivation of the E-cadherin-mediated invasion suppressor system was considered to result from reduced expression of E-cadherin in vivo (Figure 1) ▶ . Significant correlations between abnormalities of E-cadherin expression and the clinical outcome of patients with cancers have been reported. 23,24
With respect to mechanisms that regulate E-cadherin expression, footprinting analysis revealed that the positive regulatory elements of the E-cadherin promoter were bound by transcription factors in cells that expressed E-cadherin but not in those that did not. 25 DNase I hypersensitive site mapping indicated that loss of this transcriptional factor binding resulted in chromatin rearrangement in the regulatory region of the E-cadherin gene. 25 However, as far as carcinoma cells are concerned, not all E-cadherin-inactivated cancer cells were accompanied by low promoter activity, assessed by the chloramphenicol acetyltransferase assay, and therefore, CpG methylation around the promoter region was considered a possible mechanism of E-cadherin gene inactivation in human cancers. 26,27 CpG methylation around the promoter region of the E-cadherin gene and induction of E-cadherin expression following treatment with the DNA methyltransferase inhibitor 5-azacytidine were demonstrated in human cancer cell lines lacking E-cadherin expression. 26 Recently, it was discovered that some tumor suppressor genes, including RB, VHL, p15, and p16, were inactivated as a result of reduced expression due to CpG methylation (reviewed in Ref. 28 ). A new candidate tumor suppressor gene, HIC-1, was isolated by molecular analysis of a DNA site that is hypermethylated in cancer cells. 29 As observed with these tumor suppressor genes, the E-cadherin invasion suppressor gene in human cancers is silenced by an epigenetic mechanism, DNA hypermethylation.
The CpG methylation status in vivo of primary hepatocellular carcinomas and their corresponding liver tissues showing chronic hepatitis or cirrhosis, which are widely considered to be precancerous conditions, were assessed by digesting DNA with methylation-sensitive and -nonsensitive restriction enzymes. CpG methylation around the promoter region of the E-cadherin gene was detected frequently in liver tissues showing chronic hepatitis or cirrhosis and the incidence and the degree of CpG methylation increased as these precancerous conditions progressed to hepatocellular carcinomas. 30 Heterogeneous E-cadherin expression in liver tissues showing chronic hepatitis or cirrhosis, which was not observed in normal liver tissues, may be due, at least in part, to CpG methylation around the promoter region of the E-cadherin gene. 30 Immunohistochemical experiments revealed the CpG methylation around the promoter region correlated significantly with reduced E-cadherin expression in hepatocellular carcinomas. 30 Furthermore, DNA methyltransferase (EC 2.1.1.37) mRNA expression level of liver tissues showing chronic hepatitis or cirrhosis was significantly higher than that of normal liver tissues and that of hepatocellular carcinomas showed a slight further increase. 31 CpG methylation around the promoter region, which is accompanied by increased DNA methyltransferase expression, may participate in hepatocarcinogenesis, even during the early developmental stages of hepatocellular carcinomas, by reducing E-cadherin expression with consequent impairment of cell-cell adhesiveness and destruction of tissue morphology (Figure 1) ▶ .
Alterations of catenin expression in human cancers have also been reported. Expression of α-catenin was often reduced in diffuse-type human cancers, 32,33 indicating an association between down-regulation of α-catenin expression and a morphologically invasive tendency in vivo (Figure 1) ▶ . Indeed, α-catenin expression correlated significantly with a poor prognosis in patients with esophageal squamous cell carcinomas. 33
Inactivation of the E-Cadherin-Mediated Cell Adhesion System by Tyrosine Phosphorylation of β-Catenin
Another process that contributes to inactivation of the E-cadherin system is aberrant tyrosine phosphorylation of members of the cadherin-catenin complex. A rat fibroblast cell line acquired metastatic potential when transfected with v-src. 34 Normal fibroblasts formed compacted aggregates of cells that were firmly connected to each other, whereas the transformed cells were more loosely associated and could migrate from the colonies. Treatment with a tyrosine kinase inhibitor induced tight cell-cell adhesion in the aggregates of the transformed cells, whereas a tyrosine phosphatase inhibitor inhibited the cadherin-mediated aggregation of transformed cells but had little effect on that of normal fibroblasts, and β-catenin in these transformed cells was strongly tyrosine phosphorylated. 34 Therefore, tyrosine phosphorylation of β-catenin may affect the function of the cadherin system, causing cell-cell adhesion instability.
With respect to cancer cells, strong tyrosine phosphorylation of β-catenin and weak tyrosine phosphorylation of E-cadherin were observed in loosely adherent cancer cell lines, which had no mutations and did not show reduced expression of E-cadherin or α- and β-catenins. 35 Then, attention turned to looking for kinases that participated in the aberrant tyrosine phosphorylation in cancers. A 185-kd phosphorylated protein, identified as the c-erbB-2 gene product, co-immunoprecipitated with the E-cadherin-catenin complex. 36 Direct interaction between both β-catenin and plakoglobin and the c-erbB-2 gene product was confirmed by far-Western blotting analysis and using a protein-protein precipitation analysis. 37 This observation provided the first evidence that oncogene products and cell-cell adhesion molecules interact 38 and this direct interaction was proven to be mediated by the c-erbB-2 gene product core region, which is highly homologous with the epidermal growth factor receptor. Tyrosine phosphorylation of catenins was found to be initiated by epidermal growth factor, 39 which is known to induce scattering of cancer cell lines, and a direct interaction between epidermal growth factor receptors and β-catenin has also been identified. 40
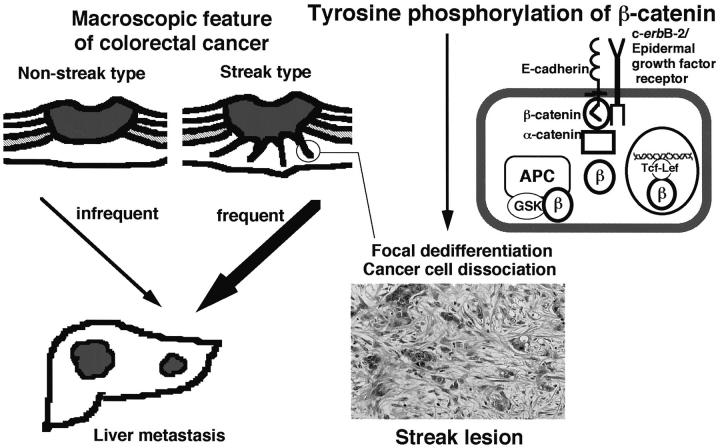
Liver metastasis is the gravest prognostic factor in patients with colorectal cancers. In an attempt to identify a reliable indicator of liver metastasis, the macroscopic features of colorectal cancers were divided into streak and nonstreak types according to the presence or absence of white streaks, which correspond to the “infiltrating” type lesions defined in the previously described histological classification system in invasive margins (Figure 2) ▶ . 41 The frequency of liver metastasis was significantly higher in patients with streak type than in patients with nonstreak type tumors and recurrent liver metastasis was associated significantly with the macroscopic features. 42 The white streaks corresponded histologically with cancer cells showing focal dedifferentiation with marked stromal fibrosis extending toward the serosa or adventitia. Focal dedifferentiation was mainly identified by the morphology of the cancer cells dissociating from the glandular structure and invading to form solitary or trabecular nests (Figure 2) ▶ . 43 It was assumed that aberrant tyrosine phosphorylation of β-catenin, a non-mutational mechanism of E-cadherin system inactivation, was probably important for the formation of streak lesions showing focal dedifferentiation in vivo. Protein extracts from streaks, cancerous tissues other than streaks and normal colonic mucosae were examined by Western blot analysis using an anti-phosphotyrosine monoclonal antibody after immunoprecipitation with an anti-β-catenin monoclonal antibody. Strong tyrosine phosphorylation of β-catenin was detected in streaks, but in neither the other cancerous tissues nor normal colonic mucosae (unpublished observation). These findings indicate that tyrosine phosphorylation of β-catenin actually participates in focal dedifferentiation in streak lesions, resulting in invasion and metastasis in vivo.
Figure 2.
Tyrosine phosphorylation of β-catenin, which transiently inactivates the E-cadherin-mediated cell adhesion system and may stimulate the APC-β-catenin-Tcf (Wingless-Wnt signaling) pathway, in streak lesions at the invading front of a colorectal cancer.
To determine the significance of tyrosine phosphorylation of β-catenin in cancers, the effects of interfering with the interaction between β-catenin and c-erbB-2 on the invasive and metastatic phenotypes of cancers were investigated. In human stomach cancer cell lines, N-terminally deleted β-catenin, which binds to c-erbB-2 but not to cadherin, inhibited the association between endogenous β-catenin and c-erbB-2 protein and suppressed the tyrosine phosphorylation of β-catenin. 44 Cells expressing N-terminally deleted β-catenin exhibited markedly reduced invasiveness in vitro and peritoneal metastasis in vivo, when inoculated into nude mice, and developed a well differentiated epithelial morphology. 44 These results suggest that tyrosine phosphorylation of β-catenin regulated by c-erbB-2 protein or other receptor-type tyrosine kinases plays an important role in the invasion, metastasis and morphogenesis of cancer cells and that inhibition of the aberrant tyrosine phosphorylation of β-catenin may be an effective means of preventing invasion and metastasis of cancer cells in vivo. Moreover, the drastically altered morphologies of these transfectants compared with the parental cells led us to consider that modulation of β-catenin may also alter the transcription of multiple genes for molecules that participate in the determination of the biological properties of cancer cells.
β-Catenin in the Wingless-Wnt Signal Transduction System
The structure of β-catenin is remarkably similar to that of the Armadillo protein of the fruit fly Drosophila, an important element in the Wingless-Wnt signaling pathway. 45,46 Wingless is a cell-cell signal in Drosophila that triggers many key developmental processes and Wnt is its analogous molecule in vertebrates. Many components of their signal transduction pathway were identified by genetic screening of Drosophila for gene products that control embryonic pattern formation. In addition to Wingless, screening yielded mutations in Porcupine, Dishevelled, Zeste white 3, and Armadillo, all encoding components of the Wingless pathway. In Xenopus laevis, homologs of Dishevelled, Zeste white 3 glycogen synthetase kinase 3β (GSK3β), and Armadillo (β-catenin) mediate Wnt signaling during dorsal ventral patterning. Direct associations between β-catenin and members of the Tcf family and Lef-1 transcription factors were reported to result in transfer of signals to the nuclei. 47 Such associations suggested that β-catenin activates transcription by forming complexes with members of the Tcf-Lef family.
β-Catenin 48,49 and γ-catenin 35 interacted directly with APC tumor suppressor gene product. GSK3β bound to APC protein and phosphorylated it in a region of the protein that can down-regulate β-catenin. 50 Adenomas and adenocarcinomas with mutant APC protein lacking the region that interacts with β-catenin from familial adenomatous polyposis patients showed increased β-catenin expression levels and both nuclei and cytoplasm showed β-catenin immunoreactivity. 51 Normal APC protein down-regulated the transcriptional activity of the β-catenin-Tcf complex and the APC-β-catenin-Tcf pathway was found to be critical for carcinogenesis: 52,53 mutations of either the APC or the β-catenin gene were found in the majority of colorectal cancers and some melanoma cell lines and ectopic expression of wild-type APC eliminated excess β-catenin from APC-defective melanoma cells. These findings indicate that APC plays an essential role in clearing unnecessary β-catenin from the cytoplasm and that β-catenin acquires oncogenic activity when it is mutated or when it is up-regulated as a result of inactivation of APC. 54
Perspectives
Inactivation of the E-cadherin system by multiple mechanisms, including both genetic and epigenetic events, plays a significant role in both the early and later stages of multistage carcinogenesis. Cadherin system inactivation may increase the pool of free and/or phosphorylated, stabilized β-catenin, thereby stimulating the Wingless-Wnt signaling pathway. Whether tyrosine phosphorylation of β-catenin by c-erbB-2 protein or other receptor-type tyrosine kinases is involved in the Wingless-Wnt signaling pathway needs to be clarified to elucidate the entire picture of the cross-talking mechanisms among cell adhesion molecules and oncogene and tumor suppressor gene products in human cancers.
Footnotes
Address reprint requests to Dr. Setsuo Hirohashi, Pathology Division, National Cancer Center Research Institute, 1-1, Tsukiji 5-chome, Chuo-ku, Tokyo 104, Japan. E-mail: shirohas@ncc.go.jp.
References
- 1.Coman DR: Decreased mutual adhesiveness, a property of cells from squamous cell carcinomas. Cancer Res 1944, 4:625-629 [Google Scholar]
- 2.McCutcheon M, Coman DR, Moore FB: Adhesiveness of malignant cells in various human adenocarcinomas. Cancer 1948, 1:460-467 [DOI] [PubMed] [Google Scholar]
- 3.Liotta LA, Steeg PS, Stetler-Stevenson WG: Cancer metastasis and angiogenesis: an imbalance of positive and negative regulation. Cell 1991, 64:327-336 [DOI] [PubMed] [Google Scholar]
- 4.Takeichi M: The cadherins: Cell-cell adhesion molecules controlling animal morphogenesis. Development 1988, 102:639-655 [DOI] [PubMed] [Google Scholar]
- 5.Takeichi M: Cadherin cell adhesion receptor as a morphogenetic regulator. Science 1991, 251:1451-1455 [DOI] [PubMed] [Google Scholar]
- 6.Behrens J, Mareel MM, Van Roy FM, Birchmeier W: Dissecting tumor cell invasion: epithelial cells acquire invasive properties after the loss of uvomorulin-mediated cell-cell adhesion. J Cell Biol 1989, 108:2435-2447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vleminckx K, Vakaet L, Jr, Mareel M, Fiers W, van Roy F: Genetic manipulation of E-cadherin expression by epithelial tumor cells reveals an invasion suppressor role. Cell 1991, 66:107-119 [DOI] [PubMed] [Google Scholar]
- 8.Mareel MM, Behrens J, Birchmeier W, De Bruyne GK, Vleminckx K, Hoogewijs A, Fiers WC, Van Roy FM: Down-regulation of E-cadherin expression in Madin Darby canine kidney (MDCK) cells inside tumors of nude mice. Int J Cancer 1991, 47:922-928 [DOI] [PubMed] [Google Scholar]
- 9.Shimoyama Y, Nagafuchi A, Fujita S, Gotoh M, Takeichi M, Tsukita S, Hirohashi S: Cadherin dysfunction in a human cancer cell line: possible involvement of loss of α-catenin expression in reduced cell-cell adhesiveness. Cancer Res 1992, 52:5770-5774 [PubMed] [Google Scholar]
- 10.Oda T, Kanai Y, Shimoyama Y, Nagafuchi A, Tsukita S, Hirohashi S: Cloning of the human α-catenin cDNA and its aberrant mRNA in a human cancer cell line. Biochem Biophys Res Commun 1993, 193:897-904 [DOI] [PubMed] [Google Scholar]
- 11.Oda T, Kanai Y, Oyama T, Yoshiura K, Shimoyama Y, Birchmeier W, Sugimura T, Hirohashi S: E-cadherin gene mutations in human gastric carcinoma cell lines. Proc Natl Acad Sci USA 1994, 91:1858-1862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oyama T, Kanai Y, Ochiai A, Akimoto S, Oda T, Yanagihara K, Nagafuchi A, Tsukita S, Shibamoto S, Ito F, Takeichi M, Matsuda H, Hirohashi S: A truncated β-catenin disrupts the interaction between E-cadherin and α-catenin: A cause of loss of intercellular adhesiveness in human cancer cell lines. Cancer Res 1994, 54:6282-6287 [PubMed] [Google Scholar]
- 13.Becker KF, Atkinson MJ, Reich U, Becker I, Nekarda H, Siewert JR, Hofler H: E-cadherin gene mutation provides clues to diffuse type gastric carcinomas. Cancer Res 1994, 54:3845-3852 [PubMed] [Google Scholar]
- 14.Kanai Y, Oda T, Tsuda H, Ochiai A, Hirohashi S: Point mutation of the E-cadherin gene in invasive lobular carcinoma of the breast. Jpn J Cancer Res 1994, 85:1035-1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muta H, Noguchi M, Kanai Y, Ochiai A, Nawata H, Hirohashi S: E-cadherin gene mutation in signet ring cell carcinoma of the stomach. Jpn J Cancer Res 1996, 87:843-848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gullford P, Hopkins J, Harraway J, McLeod M, Mcleod N, Harawja P, Talte H, Scoular R, Millers A, Reeve AE: E-cadherin germline mutations in familial gastric cancer. Nature 1998, 392:402-405 [DOI] [PubMed] [Google Scholar]
- 17.Morton RA, Ewing CM, Nagafuchi A, Tsukita S, Isaacs WB: Reduction of E-cadherin levels and deletion of the α-catenin gene in human prostate cancer cells. Cancer Res 1993, 53:3585-3590 [PubMed] [Google Scholar]
- 18.Hirano S, Kimoto N, Shimoyama Y, Hirohashi S, Takeichi M: Identification of a neural α-catenin as a key regulator of cadherin function and multicellular organization. Cell 1992, 70:293-301 [DOI] [PubMed] [Google Scholar]
- 19.Shimoyama Y, Hirohashi S, Hirano S, Noguchi M, Shimosato Y, Takeichi M, Abe O: Cadherin cell-adhesion molecules in human epithelial tissue and carcinomas. Cancer Res 1989, 49:2128-2133 [PubMed] [Google Scholar]
- 20.Shimoyama Y, Hirohashi S: Expression of E- and P-cadherin in gastric carcinoma. Cancer Res 1991, 51:2185-2192 [PubMed] [Google Scholar]
- 21.Shimoyama Y, Hirohashi S: Cadherin intercellular adhesion molecule in hepatocellular carcinoma: Loss of E-cadherin expression in an undifferentiated carcinoma. Cancer Lett 1991, 57:131-135 [DOI] [PubMed] [Google Scholar]
- 22.Birchmeier W, Behrens J: Cadherin expression in carcinomas: role in the formation of cell junctions and the prevention of invasiveness. Biochim Biophys Acta 1994, 1198:11-26 [DOI] [PubMed] [Google Scholar]
- 23.Bringuier PP, Umbas R, Schaafsma HE, Karthaus HFM, Debruyne FMJ, Schalken JA: Decreased E-cadherin immunoreactivity correlates with poor survival in patients with bladder tumors. Cancer Res 1993, 55:3241-3245 [PubMed] [Google Scholar]
- 24.Mattussen V, Paters HM, Schalkwijk L, Manni JJ, van’t Hof-Grootemboer B, De Mulder PHM, Ruiter DJ: E-cadherin expression in head and neck squamous-cell carcinoma is associated with clinical outcome. Int J Cancer 1993, 55:580-585 [DOI] [PubMed] [Google Scholar]
- 25.Hennig G, Behrens J, Truss M, Frisch S, Reichmann E, Birchmeier W: Progression of carcinoma cells is associated with alterations in chromatin structure and factor binding at the E-cadherin promoter in vivo. Oncogene 1995, 11:475-484 [PubMed] [Google Scholar]
- 26.Yoshiura K, Kanai Y, Ochiai A, Shimoyama Y, Sugimura T, Hirohashi S: Silencing of the E-cadherin invasion-suppressor gene by CpG methylation in human carcinomas. Proc Natl Acad Sci USA 1995, 92:7416-7419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Graff JR, Herman JG, Lapidus RG, Chopra H, Xu R, Jarrard DF, Isaacs WB, Pitha PM, Davidson NE, Baylin SB: E-cadherin expression is silenced by DNA hypermethylation in human breast and prostate carcinomas. Cancer Res 1995, 55:5195-5199 [PubMed] [Google Scholar]
- 28.Jones PA: DNA methylation errors and cancer. Cancer Res 1996, 56:2463-2467 [PubMed] [Google Scholar]
- 29.Wales MM, Biel MA, Deiry WE, Nelkin BD, Issa JP, Cavenee WK, Kuerbitz SJ, Baylin SB: p53 activates expression of HIC-1, a new candidate tumor suppressor gene on 17p13.3. Nature Med 1995, 1:570-577 [DOI] [PubMed] [Google Scholar]
- 30.Kanai Y, Ushijima S, Hui A-M, Ochiai A, Tsuda H, Sakamoto M, Hirohashi S: The E-cadherin gene is silenced by CpG methylation in human hepatocellular carcinomas. Int J Cancer 1997, 71:355-359 [DOI] [PubMed] [Google Scholar]
- 31.Sun L, Hui A-M, Kanai Y, Sakamoto M, Hirohashi S: Increased DNA methyltransferase expression is associated with an early stage of human hepatocarcinogenesis. Jpn J Cancer Res 1997, 88:1165-1170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ochiai A, Akimoto S, Shimoyama Y, Nagafuchi A, Tsukita S, Hirohashi S: Frequent loss of α catenin expression in scirrhous carcinomas with scattered cell growth. Jpn J Cancer Res 1994, 85:266-273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakanishi Y, Ochiai A, Akimoto S, Kato H, Watanabe H, Tachimori Y, Yamamoto S, Hirohashi S: Expression of E-cadherin, α-catenin, β-catenin and plakoglobin in esophageal carcinomas and its prognostic significance. Oncology 1997, 54:158-165 [DOI] [PubMed] [Google Scholar]
- 34.Matsuyoshi N, Hamaguchi M, Taniguchi S, Nagafuchi A, Tsukita S, Takeichi M: Cadherin-mediated cell-cell adhesion is perturbed by v-src tyrosine phosphorylation in metastatic fibroblasts. J Cell Biol 1992, 118:703-714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shibata T, Gotoh M, Ochiai A, Hirohashi S: Association of plakoglobin with APC, a tumor suppressor gene product, and its regulation by tyrosine phosphorylation. Biochem Biophys Res Commun 1994, 203:519-522 [DOI] [PubMed] [Google Scholar]
- 36.Ochiai A, Akimoto S, Kanai Y, Shibata T, Oyama T, Hirohashi S: c-erbB-2 gene product associates with catenins in human cancer cells. Biochem Biophys Res Commun 1994, 205:73-78 [DOI] [PubMed] [Google Scholar]
- 37.Kanai Y, Ochiai A, Shibata T, Oyama T, Ushijima S, Akimoto S, Hirohashi S: c-erbB-2 gene product directly associates with β-catenin and plakoglobin. Biochem Biophys Res Commun 1995, 208:1067-1072 [DOI] [PubMed] [Google Scholar]
- 38.Klymkowsky MW, Parr B: The body language of cells: the intimate connection between cell adhesion and behavior. Cell 1995, 83:5-8 [DOI] [PubMed] [Google Scholar]
- 39.Shibamoto S, Hayakawa M, Takeuchi K, Hori T, Oku N, Miyazawa K, Kitamura N, Takeichi M, Ito F: Tyrosine phosphorylation of β-catenin and plakoglobin enhanced by hepatocyte growth factor and epidermal growth factor in human carcinoma cells. Cell Adhesion Commun 1994, 1:295-305 [DOI] [PubMed] [Google Scholar]
- 40.Hoschuetzky H, Aberle H, Kemler R: β-Catenin mediates the interaction of the cadherin-catenin complex with epidermal growth factor receptor. J Cell Biol 1994, 127:1375-1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jass JR, Love SB, Northover JM: A new prognostic classification of rectal cancer. Lancet 1987, 1:1303-1306 [DOI] [PubMed] [Google Scholar]
- 42.Inomata F, Ochiai A, Sugihara K, Moriya Y, Yamaguchi N, Adachi Y, Kitano S, Hirohashi S: Macroscopic features at the deepest site of tumor penetration predicting liver metastases of colorectal cancer. Jpn J Clin Oncol 1998, 28:123-128 [DOI] [PubMed] [Google Scholar]
- 43.Ono M, Sakamoto M, Ino Y, Moriya Y, Sugihara K, Muto T, Hirohashi S: Cancer cell morphology at the invasive front and expression of cell adhesion-related carbohydrate in the primary lesion of patients with colorectal carcinoma with liver metastasis. Cancer 1996, 78:1179-1186 [DOI] [PubMed] [Google Scholar]
- 44.Shibata T, Ochiai A, Kanai Y, Akimoto S, Gotoh M, Yasui N, Machinami R, Hirohashi S: Dominant negative inhibition of the association between β-catenin and c-erbB-2 by N-terminally deleted β-catenin suppresses the invasion and metastasis of cancer cells. Oncogene 1996, 13:883-889 [PubMed] [Google Scholar]
- 45.Hinck L, Nelson WJ, Papkoff J: Wnt-1 modulates cell-cell adhesion in mammalian cells by stabilizing β-catenin binding to the cell adhesion protein cadherin. J Cell Biol 1994, 124:729-741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peifer M: Regulating cell proliferation: as easy as APC. Science 1996, 272:974-975 [DOI] [PubMed] [Google Scholar]
- 47.Behrens J, von Kries JP, Kuhl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W: Functional interaction of β-catenin with the transcription factor LEF-1. Nature 1996, 382:638-642 [DOI] [PubMed] [Google Scholar]
- 48.Rubinfeld B, Souza B, Albert I, Muller O, Chamberlain SH, Masiarz FR, Munemitsu S, Polakis P: Association of the APC gene product with β-catenin. Science 1993, 262:1731-1734 [DOI] [PubMed] [Google Scholar]
- 49.Su LK, Vogelstein B, Kinzler KW: Association of the APC tumor suppressor protein with catenins. Science 1993, 262:1734-1737 [DOI] [PubMed] [Google Scholar]
- 50.Rubinfeld B, Albert I, Porfiri E, Fiol C, Munemitsu S, Polakis P: Binding of GSK3β to the APC-β-catenin complex and regulation of complex assembly. Science 1996, 272:1023-1026 [DOI] [PubMed] [Google Scholar]
- 51.Inomata M, Ochiai A, Akimoto S, Kitano S, Hirohashi S: Alteration of β-catenin expression in colonic epithelial cells of familial adenomatous polyposis patients. Cancer Res 1996, 56:2213-2217 [PubMed] [Google Scholar]
- 52.Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler KW: Activation of β-catenin-Tcf signaling in colon cancer by mutations in β-catenin or APC. Science 1997, 275:1787-1790 [DOI] [PubMed] [Google Scholar]
- 53.Rubinfeld B, Robbins P, El-Gamil M, Albert I, Porfiri E, Polakis P: Stabilization of β-catenin by genetic defects in melanoma cell lines. Science 1997, 275:1790-1792 [DOI] [PubMed] [Google Scholar]
- 54.Nakamura Y: Cleaning up on β-catenin. Nat Med 1997, 3:499-500 [DOI] [PubMed] [Google Scholar]