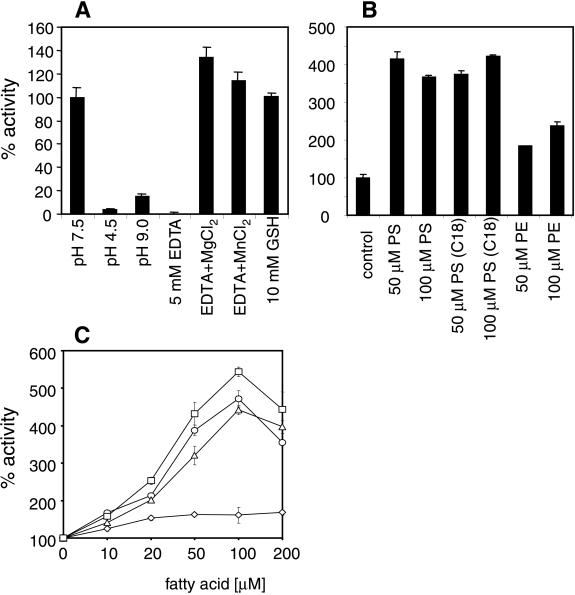
Figure 2.
Enzymatic properties of murine nSMase2. (A) pH optimum and ion requirement of murine nSMase2 from membrane extracts of stably transfected HEK 293 cells. Control assay mixtures contain 5 mM MgCl2, 0.05% Triton X-100, 5 mM DTT, 100 mM Tris⋅Cl at pH 7.4, and a protease inhibitor mixture (1× Complete without EDTA; Roche Molecular Biochemicals). Measurements with EDTA and metal ions were done with 5 mM EDTA and 10 mM of the respective metal ion. (B) Influence of phospholipids on nSMase2 activity. Phospholipids were dissolved in 0.05% Triton X-100 by sonication and premixed with the assay components before adding the enzyme preparation. Results represent mean values (±SD) from three independent measurements. PS, phosphatidylserine; PS(C18), dioleoyl-PS; PE, phosphatidylethanolamine. (C) Activation of nSMase2 activity by unsaturated fatty acids. Fatty acids, dissolved in EtOH at various concentrations, were added directly to the assay mixture. ⋄, Stearic acid; ▵, linolenic acid; ○, linoleic acid; and □, arachidonic acid.