Abstract
Severe intrauterine growth restriction (IUGR) is characterized by abnormal placentation. Mouse gene knockout studies show that an absence of either hepatocyte growth factor (HGF) or its receptor, c-met, leads to intrauterine death secondary to severe IUGR with deficient placentation. In this study, immunocytochemistry localized HGF protein throughout placental villi across gestation, whereas c-met protein was localized only to the perivillous trophoblast and vascular endothelium. Within the IUGR placentae, a reduction in HGF immunostaining within the villous stroma was observed. HGF mRNA was strongly expressed in the perivascular tissue around the stem villous arteries throughout gestation, with weaker expression within the villous stroma and the terminal villi. c-met mRNA expression was limited to the perivillous trophoblast, particularly in the first trimester, with only a faint hybridization signal from the villous stroma. Placental mRNA expression was examined quantitatively using a ribonuclease protection assay: HGF and c-met mRNA expression increased from the first to the second trimester, reaching a zenith before decreasing again through the third trimester to term. HGF mRNA levels were significantly reduced in the IUGR placentae (P = 0.036), whereas c-met mRNA expression was within the normal range for gestation. These findings suggest that HGF derived from the perivascular tissue of stem villous arteries may play an important role in controlling normal villous development. Whereas reduced expression of HGF within IUGR placentae does not prove a causative link with abnormal villous development, the association lends support to this possibility.
Intrauterine growth restriction (IUGR) remains a major cause of perinatal morbidity and mortality. A specific cohort of pregnancies with IUGR secondary to uteroplacental insufficiency is particularly at risk because of poor placental function. 1 These pregnancies are characterized by abnormal placentation, abnormal umbilical artery diastolic velocities, and reduced liquor. 2 The placentae from these pregnancies are significantly smaller than those of controls, with a diminished mean placental surface area and specific morphological abnormalities, including reduced cytotrophoblast proliferation, smaller terminal villi, and abnormal villous vasculature. 3
Hepatocyte growth factor (HGF) is a secreted heparin-binding glycoprotein that was originally identified from rat platelets on the basis of its ability to stimulate mitogenesis in hepatocytes. 4 It is now known to be a multifunctional cytokine with angiogenic properties, which variously regulates growth, motility, and morphogenesis in receptive cells. 5-7 All these functions are potentially important in placentation, in which proliferation and cellular orientation of trophoblast, mesenchyme, and vascular endothelia are required.
HGF is secreted as an inactive, single-chain 92-kd glycoprotein by cells of embryological mesenchymal origin. The active form, a heterodimer consisting of a heavy α chain (55–65 kd) and light β chain (32–36 kd), results from proteolytic cleavage of the precursor, which is thrombin dependent. 8,9
The biological effects of HGF are mediated by its high-affinity receptor, c-met, a trans-membranous protein encoded by the MET proto-oncogene. 10 c-met undergoes proteolytic cleavage before expression on the cell surface as a heterodimeric protein. It consists of an extracellular 50-kd α chain and a 145-kd β chain that has extracellular, trans-membranous, and intracellular regions. The intracellular domain of the β subunit contains a tyrosine kinase domain and sites for autophosphorylation through which the cellular effects of c-met are mediated. 10,11 c-met is expressed by many epithelial cell types, as well as vascular endothelium. 12
The biological activities and expression of HGF and its receptor in embryonic life suggest an important role for them in mesenchymal-epithelial interactions during organogenesis. 12-14 Direct evidence on the essential role of HGF/c-met in embryogenesis comes from recent experiments in mice using gene knockout techniques. Mice heterozygous for the disrupted gene (HGF or c-met) were phenotypically normal, whereas those homozygous for the defect became severely growth restricted in the second trimester, resulting in universal lethality by the early third trimester. 15-17 The placentae of mutant embryos were much smaller than normal and had a poorly developed labyrinthine region. The key abnormality was severe depletion of the labyrinthine trophoblast cells, homologous to the perivillous trophoblast in human placentae, combined with poor vascularization of this region.
HGF and c-met mRNA and protein have previously been localized in both first trimester and term human placentae. 18-20 In this study, we report for the first time their cellular localization and quantitative expression in the placentae of human pregnancies throughout gestation. We also note changes in cellular distribution and expression of HGF and c-met in pregnancies complicated by severe IUGR.
Materials and Methods
Tissue Collection
Placentae were obtained from normal first, second, and third trimester pregnancies and also pregnancies complicated by severe IUGR (see Table 1 ▶ ). First and second trimester placentae were obtained after surgical termination of pregnancy; other placentas were obtained after nonlaboring cesarean section. Growth-restricted fetuses were identified prospectively using ultrasound, and the diagnosis used in our study was based on the following criteria: 1) either an estimated fetal weight of below the third centile for gestational age or serial growth measurements indicating decreased growth velocity >1.5 standard deviations, 2) absent or significantly reduced umbilical artery end diastolic blood flow, and 3) reduced liquor volume. 21
Table 1.
Clinical Details of Pregnancies from Which Placenta Were Examined
| Trimester | IUGR | Gestation matched | |||
|---|---|---|---|---|---|
| First | Second | Third | |||
| Number (n) | 8 | 5 | 15 | 6 | 9 |
| Gestation (weeks) | 11 (9–13) | 16 (14–17) | 37 (27–41) | 31 (26–38) | 32 (27–38) |
| Birth weight (g) | NA | NA | 2905 (1100–3860) | 1083 (595–2060) | 1700 (1084–3360) |
| AEDF | NA | NA | 0 | 5 | 0 |
| Oligohydramnious | 0 | 0 | 0 | 6 | 0 |
Median (range) gestational age and birth weight are displayed. AEDF, number with absent end diastolic flow (in the umbilical artery); oligohydramnios, number with maximum depth of liquor <2 cm; NA, not applicable.
Full-thickness placental biopsies were taken from a central location lying between the basal and chorionic plates. The samples of fresh placental tissue were divided into small pieces and thoroughly washed in phosphate-buffered 0.9% saline (PBS). Tissues were variously preserved: for immunocytochemistry, biopsies were fixed in Formalin before embedding in paraffin wax; for in situ hybridization, they were fixed in 4% paraformaldehyde for 24 hours before being washed, snap frozen in Cryo-Embed (Bright Instrument Co., Huntingdon, UK) and stored at −70°C; for protein or RNA extraction, they were snap frozen in liquid nitrogen immediately after collection and stored at −70°C. Ethical approval for tissue collection was obtained from the South Birmingham Ethical Committee.
Western Immunoblotting
Total protein was extracted from snap-frozen placental tissue using a lysis buffer (50 mmol/L Tris-HCl (pH 8.0), 1% Triton X-100, 0.1% sodium dodecyl sulfate, 250 mmol/L NaCl, and 5 mmol/L ethylenediaminetetraacetic acid) containing 40 mg/ml phenylmethysulfonyl fluoride, 5 mg/ml pepstatin, and 5 mg/ml leupeptin. Protein concentration was calculated spectrophotometrically at 595 nm using a Coomassie blue reagent and human albumin standards. Analysis was performed using sodium dodecyl sulfate-polyacrylamide gel electrophoresis on discontinuous acrylamide gels according to the method of Laemmli, 22 using the Mini Protean II System (Bio-Rad, Herts, UK). One hundred micrograms of protein was applied per lane under reducing conditions, and biotinylated molecular weight markers (Bio-Rad) were run in parallel lanes.
After electrophoresis, proteins were transferred to nitrocellulose membranes, which were then blocked for nonspecific binding with a solution containing 5% nonfat milk, 0.1% bovine serum albumin, 0.1% Tween 20, and PBS. Membranes were incubated with a primary antibody (anti-HGF (1 in 2000): rabbit polyclonal antibody raised against human HGF, supplied by Dr T. Ishii (Mitsubushi Kasei Corp., Yokohamu, Japan); anti-c-met (1 in 500): affinity-purified rabbit polyclonal antibody raised against a peptide that corresponds to the C-12 terminal amino acids of the human c-met p140 (anti h-met) (Santa Cruz Biotechnology Inc., Santa Cruz, CA)). Antibody binding was visualized using a secondary antibody (peroxide-conjugated goat anti-rabbit (1 in 2000): Dako Ltd., Bucks, UK) with an enhanced chemiluminescence system (Amersham International, Bucks, UK) and recorded by exposure to preflashed film (Hyperfilm-ECL, Amersham International). The specificity of secondary antibody binding was tested by performing no-primary controls, which were negative (data not shown). The biotinylated molecular weight markers were visualized separately by incubating with an avidin-horseradish peroxidase complex (Bio-Rad, Richmond, CA) followed by enhanced chemiluminescence as above.
Immunocytochemistry
Immunocytochemistry was performed as described previously. 18 Serial 3-μm sections of Formalin-fixed, paraffin-embedded tissue were deparaffinized with xylene and then rehydrated, and endogenous peroxidase activity was quenched by the addition of 0.3% (v/v) hydrogen peroxide. Primary antibodies were as above, anti-HGF (1 in 25) and anti c-met (1 in 5). Amplification of the primary antibody reaction was achieved by incubation with an appropriate secondary antibody, followed by a complex of streptavidin and biotinylated peroxidase. The binding was visualized by incubating the sections with diaminobenzidine and hydrogen peroxide in PBS. Sections were counterstained with Mayer’s hematoxylin, dehydrated, and mounted. Negative control reactions were performed by omitting the primary antibodies and also, for anti-HGF, by preabsorbing the primary antibody with an excess of recombinant HGF.
Ribonuclease Protection Assay
Total RNA was extracted from snap-frozen placental tissue by the guanidinium thiocyanate/acid phenol extraction method and quantified spectrophotometrically at 260 nm. 23 [32P]CTP-labeled anti-sense riboprobes (specific activity ∼4 × 10 8 cpm/μl) for HGF (677 bp) and c-met (500 bp) were constructed from plasmid-derived cDNAs that we have described previously. 18 The pTRI-β-actin control template (Ambion, Whitney, UK) was used to construct a 298-bp [32P]CTP-labeled anti-sense riboprobe (specific activity ∼5 × 10 7 cpm/μl) corresponding to a fragment of human cytoplasmic actin. This was used as a constitutively expressed gene product to control for differences in RNA loading, allowing comparison of HGF and c-met mRNA expression between RNA samples run on the same gel. A total of 34 placental samples were analyzed on five gels; to allow comparison of results between gels, a minimum of 4 identical placental control samples were run on each gel. The assay was performed as described previously. 24
Sample RNA (20 μg) was mixed with the three riboprobes (HGF and c-met, ∼1 × 10 5 cpm; β-actin, ∼2 × 10 4 cpm), precipitated with ethanol and redissolved in hybridization buffer. Samples were then denatured at 90°C and incubated overnight at 45°C. Nonhybridized RNA was removed by incubating with an RNase digestion buffer, which was subsequently inactivated by the addition of proteinase K. After phenol-chloroform extraction and ethanol precipitation, the RNA was resuspended in loading buffer, denatured at 85°C, and electrophoresed on a polyacrylamide/urea gel. The gels were then washed, transferred to chromatography paper, and dried under vacuum. Autoradiography was performed using preflashed X-ray film (Hyperfilm MP, Amersham International) with intensifying screens at −70°C for 1 to 6 days. Quantification of autoradiographic signals was performed by densitometric analysis (area under the curve), and transcripts detected were assigned relative densitometric units. The ratio of HGF/actin and c-met/actin was then calculated for each sample. A positive and negative control reaction was carried out for each experiment using yeast tRNA in the presence and absence of the RNase digestion buffer.
In Situ Hybridization
In situ hybridization for both HGF and c-met was performed on 6-μm sections of paraformaldehyde-fixed, frozen tissues as previously described. 18 [35S]UTP-labeled riboprobes (both sense and anti-sense) were synthesized from a 677-bp cDNA for HGF and a 500-bp cDNA for c-met. Sense and anti-sense hybridizations were performed on serial sections, with the sense riboprobe acting as a negative control to demonstrate any nonspecific hybridization. The hybridized sections were dipped into radiographic emulsion (Ilford K5, Mobberley, UK) and stored at 4°C for 14 to 21 days, before developing and counterstaining with hematoxylin and eosin.
Statistics
Normal distribution of results was not assumed. The Kruskal-Wallis test was used to compare groups of medians, Dunn’s multiple comparison test for posthoc analysis, and the Mann-Whitney test to compare unpaired groups. All calculations were performed using a computer analysis package (Prism 2.0, GraphPad Software, San Diego, CA).
Results
Table 1 ▶ summarizes the clinical data from early gestational, uncomplicated third trimester, and IUGR groups.
Cellular Localization of HGF and c-met
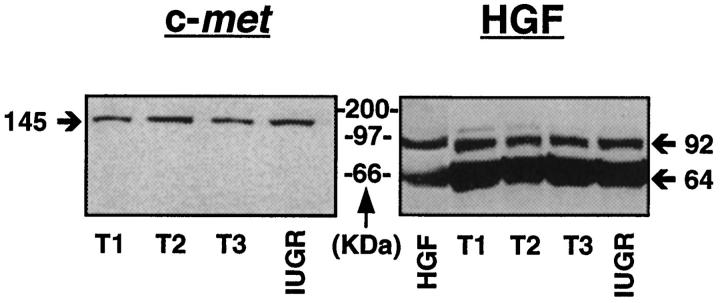
Western immunoblotting using protein lysates from placental tissue under reducing conditions revealed bands of appropriate sizes for HGF and c-met throughout gestation and in the presence of IUGR, confirming specificity of the antibodies (Figure 1) ▶ .
Figure 1.
Autoradiographs of Western immunoblots run under reducing conditions using placental protein lysates from first (T1), second (T2), and third (T3) trimesters and IUGR. The HGF antibody reveals a major band of ∼64 kd, equivalent to the α chain of HGF, with a weaker band at ∼92 kd, equivalent to unreduced dimeric HGF. Bands of similar size are seen with the recombinant active (dimeric) HGF. The h-met antibody reveals a single band of ∼145 kd, equivalent to the β chain of c-met. Under nonreducing conditions (data not shown), a single 190-kd band, equivalent to the full-length molecule, was found.
In first trimester placental tissue, the perivillous trophoblast was immunopositive to HGF, with the syncytiotrophoblast demonstrating slightly stronger staining than the cytotrophoblast (Figure 2A) ▶ . The mesenchymal core was relatively loosely structured but also showed immunoreactive staining to HGF along with the Hofbauer cells. The corresponding section immunostained with anti c-met antibody demonstrated similarly intense immunolocalization in the trophoblast layer, but with little apparent difference between cytotrophoblast and syncytiotrophoblast (Figure 2B) ▶ . Little stromal immunostaining to c-met was noted other than in the vascular endothelium. Both HGF and c-met were also immunolocalized within the decidua (Figure 2, C and D) ▶ . In control sections no positive staining was found when the primary antibody was omitted (Figure 2E) ▶ . Similarly, no immunostaining was observed when the antiserum was preabsorbed by excess of HGF (data not shown).
Figure 2.
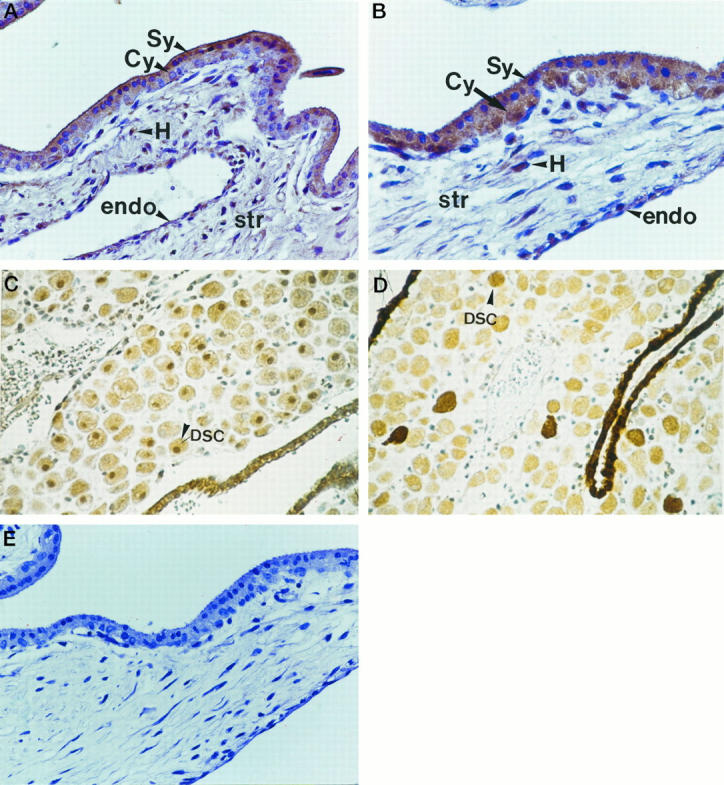
In the first trimester villus, the syncitiotrophoblast (Sy) and cytotrophoblast (Cy) layers of the villus were stained immunopositive to HGF (A) and c-met (B). The mesenchymal stromal core (str) was relatively loosely structured and stained positively for HGF along with the Hofbauer cells (H). The vascular endothelium (endo) stained weakly positive for both HGF (C) and c-met (D). Control sections (see text) were negative (E).
In normal third trimester placentae, HGF immunostaining was most intense in the fused syncytioblast/cytotrophoblast layer surrounding the villi (Figure 3A) ▶ . There was also positive immunoreactivity to HGF in the mesenchymal villous core and endothelial cells lining the vasculature. c-met immunoreactivity was strongest in the fused syncytioblast/cytotrophoblast but was also evident around vascular endothelium (Figure 3B) ▶ .
Figure 3.
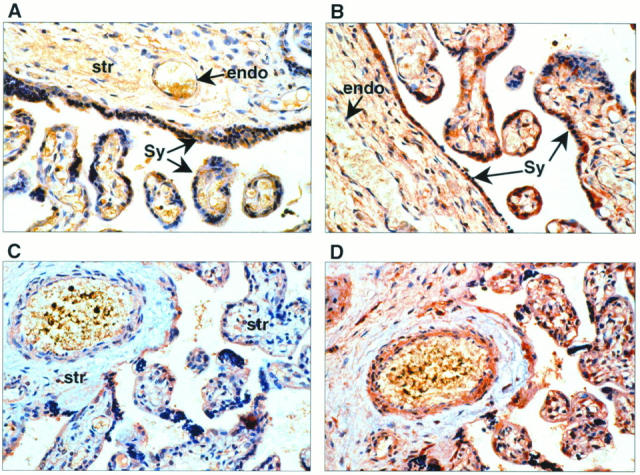
In placentae from uncomplicated third trimester pregnancies, positive cytoplasmic staining was identified using anti-HGF antibody (A), most intensely in the syncytium (Sy). There was also positive immunoreactivity to HGF in the endothelial cells (endo) lining the vasculature of the villi and the cells within the mesenchymal stromal core (str). B: c-met immunolocalization is primarily to the vascular endothelium (endo) within villi and to the syncytium (Sy) around the villi. In third trimester placentae complicated by IUGR, HGF immunoreactivity was localized in the same tissue types as in placentae of “uncomplicated” pregnancies (C). However, the HGF immunostaining intensity was less marked in the mesenchymal stroma. In serial sections immunostained for c-met, there was no discernible alteration in either localization or intensity (D).
In IUGR placentae HGF immunolocalization was similar to that in normal placentae; however, the immunostaining intensity appeared reduced in the mesenchymal stroma (Figure 3C) ▶ . A blinded operator reviewing the tissue sections qualitatively scored this (Table 2) ▶ and showed that HGF immunostaining within the stroma appeared reduced in the IUGR placentae compared with normal controls. In serial sections immunostained for c-met, no discernible alteration in localization or intensity was noted (Figure 3D) ▶ .
Table 2.
Immunostaining to HGF and c-met in Normal and IUGR Placentae
| Tissue | Immunostaining intensity | |||
|---|---|---|---|---|
| Normal (n = 6) | IUGR (n = 6) | |||
| HGF | c-met | HGF | c-met | |
| Syncytium | +++ | +++ | +++ | +++ |
| Villous stroma | +++ | − | + | − |
| Villous endothelium | ++ | ++ | ++ | ++ |
| Decidual cells | ++ | ++ | ++ | ++ |
| Hofbauer cells | +++ | − | +++ | − |
| Amnion/chorion | +++ | +++ | +++ | ++ |
One of the authors (X-FL), blinded to the origin of the sections, graded the intensity of immunostaining in each of the placental cell types. Staining was graded as intense (++++), strong (+++), moderate (++), weak (+) and absent (−) compared with the negative control section.
Expression of mRNA for HGF and c-met in Placental Tissues
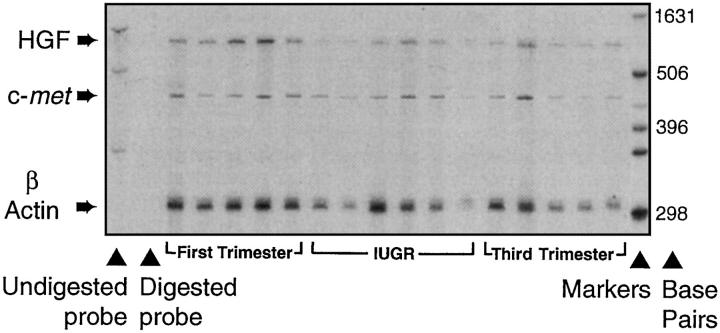
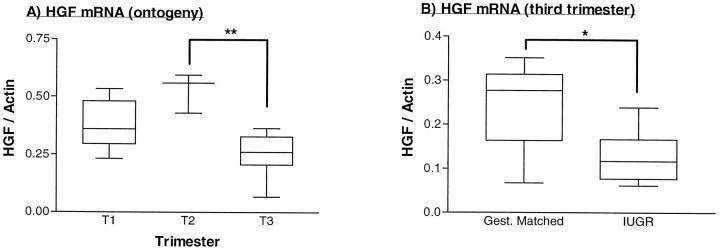
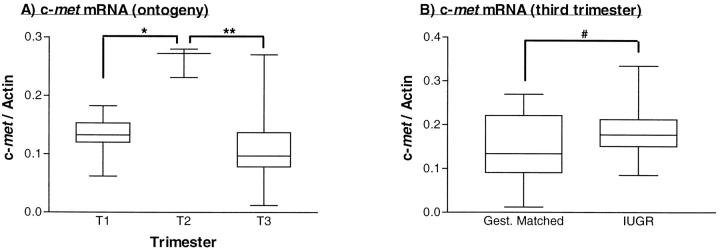
Figure 4 ▶ shows the quantification of mRNA levels in gestational placental tissues using the ribonuclease protection assay. Both HGF and c-met mRNA expression increased from the first to the second trimester, when they were maximally expressed, before falling to their lowest levels at term (Figures 5A and 6A) ▶ ▶ . Within IUGR placentae, HGF mRNA was significantly reduced when compared with gestationally matched normal controls (Figure 5B) ▶ , whereas c-met mRNA levels were within normal limits for gestation (Figure 6B) ▶ .
Figure 4.
Example of an autoradiograph of the ribonuclease protection assay. The signals of the protected fragments were quantified using computer densitometry in placentae from first, second, and third trimesters and IUGR. The actin RNA probe has been used as an internal control to correct for variations in RNA loading between lanes. The results are expressed as arbitrary optical units for HGF/actin and c-met/actin. A total of 34 placental samples were analyzed on five gels using common control samples to allow direct comparison.
Figure 5.
Quantification of HGF mRNA using the ribonuclease protection assay. A: Normal placenta from first (T1), second (T2), and third (T3) trimester placenta. P = 0.0008 (Kruskal-Wallis test, statistic = 14.28). **P < 0.001 (Dunn’s multiple comparison test, Δ rank sum = 15.40). B: IUGR and gestationally matched third trimester placenta. *P = 0.036 (Mann-Whitney test, two-tailed P value, U = 9.0). Box and whisker plots are shown, indicating medians, interquartile ranges, and total ranges.
Figure 6.
Quantification of c-met mRNA using the ribonuclease protection assay. A: Normal placenta from first (T1), second (T2), and third (T3) trimester placenta. *P = 0.006 (Kruskal-Wallis test, statistic = 10.22). **P < 0.005 (Dunn’s multiple comparison test, Δ rank sum = 11.40). B: IUGR and gestationally matched third trimester placenta. #P = 0.53 (Mann-Whitney test, two-tailed P value, U = 21.0). Box and whisker plots are shown, indicating medians, interquartile ranges, and total ranges.
In situ hybridization studies from the first trimester reveal HGF mRNA to be localized to the villous stroma, and in particular to the perivascular tissue (Figure 7, A and B) ▶ , with no signal from either cytotrophoblast or syncytiotrophoblast. Contrasting this, c-met mRNA is clearly localized to the perivillous trophoblast with little signal from the stroma (Figure 7, C and D) ▶ . In the third trimester, the localization of HGF to the perivascular tissue around the vasculature of the stem villi is clearly demonstrated, with a much weaker signal from the stroma of the terminal villi (Figure 7, E and F) ▶ . The c-met hybridization signal in the third trimester is less marked but is still visible localizing to the fused syncitioblast/cytotrophoblast membrane of both the stem and terminal villi (Figure 7, G and H) ▶ . No discernible difference in the localization of HGF and c-met mRNA in IUGR placentae was observed (data not shown).
Figure 7.
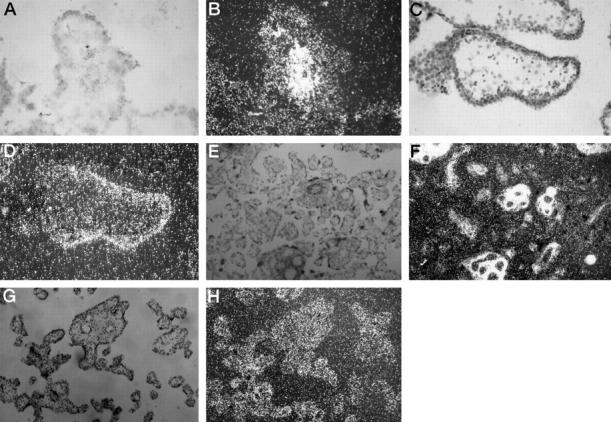
In situ hybridization studies (using antisense probes) localizing mRNA on placental sections from first and third trimesters showing light- and dark-field views. A and B: HGF in a first trimester stem villus. C and D: c-met in a first trimester stem villus. E and F: HGF in third trimester stem and terminal villi. G and H: c-met in third trimester stem and terminal villi. Duplicate sections using sense probes showed no specific hybridization (data not shown).
Discussion
HGF is a potent stimulator of both cell proliferation and DNA synthesis in receptive cells, including trophoblast and microvascular endothelium. 5,25,26 It also increases cell motility and has both chemotactic and chemokinetic properties. 27 On epithelial and endothelial cells (which often express the c-met receptor), transduction via tyrosine kinase produces increased cell division and DNA synthesis together with morphogenic effects, including the formation of branching tubular structures. 28 In vitro experiments using corneal implants have demonstrated that HGF is also a potent angiogenic factor. 25,29 Such effects would have potential biological importance if HGF were to influence placental development.
This study describes the co-localization of HGF and c-met mRNA in human placentae through gestation. Previous work identified the villous perivascular stroma as the primary site of HGF production within term placentae. 18 These results show that the perivascular stroma within immature stem villi from the 8th week of gestation onward may be an important source of HGF. As the placenta matures an intense hybridization signal surrounds the larger, muscle-walled villous arteries in the stem villi, with little signal from the terminal villi. Perivascular smooth muscle has previously been identified as a potent source of HGF, 30 and these in situ hybridization studies suggest that it may be the main source of placental HGF. The high-affinity receptor for HGF, c-met, appears to be produced in the villous trophoblast layer, and in situ hybridization studies show localization in this area throughout gestation. This signal includes the trophoblast surrounding the terminal villi in later gestation (the site of continuing villous growth and development), contrasting the localization of HGF mRNA.
Successful pregnancy depends on placental growth and development, with trophoblast proliferation, decidual interaction, and vasculogenesis being important early in gestation. 31,32 As gestation increases, maternofetal exchange of oxygen, nutrients, and waste products are optimized by a progressive increase in total villous surface area and vasculature. 33 This is matched by decreasing thickness of both trophoblast and stromal components of the fetomaternal vascular barrier. It was postulated that capillary loop elongation within intermediate villi may have a key role to play in the placental remodeling that occurs throughout gestation, 34 and this has been confirmed both stereologically and morphologically. 33
HGF protein immunolocalization was observed in the trophoblast bilayer of the villus in first trimester placentae and was particularly intense in the syncytiotrophoblast. We have previously described the cellular localization of HGF in third trimester placentae. 18 Such immunolocalization of HGF protein was reconfirmed with intense staining of the fused syncytioblast/cytotrophoblast membrane and immunolocalization within the villous core. The stromal immunostaining for HGF protein seems to increase with gestation, being most intense in normal third trimester placentae.
c-met protein immunostaining was demonstrable on both the trophoblast and the vascular endothelial cells in first and third trimester placental villi. Although localization to the endothelium was not confirmed by in situ hybridization, this finding is consistent with previous studies in placenta and other fetal and adult tissues. 12,14,29,35 The c-met immunoreactivity does not seem to alter in its pattern of localization with increasing gestation. The co-localization of c-met protein and mRNA in the trophoblast 18,19 indicates that HGF-induced DNA synthesis in this cell type may be secondary to receptor interaction, 26,36 and it is likely that the immunolocalization of HGF to these cells (and also to the decidua) represents receptor-bound antigen. It may be that HGF is a cytokine that, at least in part, mediates cytotrophoblast proliferation and differentiation. However, although HGF has been shown to stimulate DNA synthesis in primary cultured cytotrophoblasts, the production of human placental lactogen secreted from the fully differentiated syncitial layer did not increase with increasing HGF concentrations. 26 This implies that HGF may be more important in stimulating cytotrophoblast proliferation than in inducing differentiation. The localization of HGF mRNA to the perivascular stroma of the stem villi, and of HGF protein to the villous stroma and perivillous trophoblast, is consistent with a role for HGF as a secreted mitogen/morphogen acting on trophoblast cells, which express both c-met mRNA and protein. 36
In rat placentae, quantitative mRNA expression (measured by Northern blotting) for both HGF and c-met was higher at the beginning of pregnancy (day 6) than at the end. 37 Similarly, the scatter activity (in vitro assay) showed a relative decrease (per mg of protein) toward the end of gestation. In human placenta, this study has shown (using an RNase protection assay) that HGF mRNA levels increase significantly from the first to the second trimester, reaching a zenith, and then fall in the third trimester. These results are consistent with previous work reporting amniotic fluid concentrations of HGF to be maximal during the second trimester of human pregnancy. 38 The current study also demonstrates a significant increase in placental c-met mRNA expression from the first to the second trimester, when it too reaches a peak before falling toward term. The gestational age of the second trimester placental samples had a range between 14 and 17 weeks; significantly, this is the time when trophoblast proliferation and invasion, and placental angiogenesis, are at their greatest.
Recent genetic studies using homologous recombination to knock out one parental gene encoding HGF in mice have indicated that exon 5, which encodes the first kringle domain and is essential for the biological activities of HGF, is vital for placental and fetal growth in mice. 17 Homozygotes demonstrated poor fetal growth and deficient placentation with an abnormal spongeotrophoblast cell type. This ended in embryonic lethality two-thirds of the way through pregnancy. Identical results were obtained when exon 2 was disrupted. 16 These data indicate that in mice, functional HGF expression is required for normal placentation to occur.
This study describes a subjective reduction in stromal immunolocalization of HGF protein and a quantitative reduction in mRNA in human placentae from pregnancies complicated by IUGR. Factors regulating placental HGF production remain unclear. Recent studies describing microdissection of placental villi have indicated that HGF production by the villous core is reduced by removal of the covering trophoblast. 20 It is possible that the cytotrophoblast stem cells in the intact placental villi are in some way responsible for regulating villous core HGF production. However, the study in question concentrated on villous core fibroblasts as the source of HGF. Given the evidence presented here, further investigation into interactions between stem villous perivascular stroma and trophoblast is needed.
The cohort of IUGR pregnancies studied were toward the severe end of the spectrum, with the fetuses demonstrating reduced growth by the late second and early third trimester. The placentae of these pregnancies have been reported as having reduced villous tree elaboration and diminished surface area stereologically, and these findings were particularly pronounced in those subjects with abnormal Doppler velocimetry. 3 We have not proven a causative link between severe IUGR and reduced HGF expression, and it may be that this finding is merely a reflection of the abnormal villous morphology. However, the mouse gene knockout experiments do provide circumstantial evidence that HGF itself may be responsible for the maldevelopment of these placentae.
Acknowledgments
We thank the consultants and Delivery Suite staff of the Birmingham Women’s Hospital for their support in the collection of tissues. Particular thanks are due to Dr. Terry Rollason for his expert advice regarding the localization studies, Dr. Conrad Smith for his assistance with setting up the RNase protection assay, Ms. Julie Verhaeg for her assistance with the immunocytochemistry, and Ms. Janine Fear for her assistance with the in situ hybridization studies. We gratefully acknowledge the help of Prof. Lucilla Poston and Dr. Lorin Lakasing, who provided some extremely rare normal early third trimester tissue.
Footnotes
Address reprint requests to Dr. Mark D. Kilby, Department of Fetal Medicine, Birmingham Women’s Hospital, Edgbaston, Birmingham, B15 2TG, UK. E-mail: m.d.kilby@bham.ac.uk.
Supported by grants from the British Heart Foundation (PG/97056), the Wellcome Trust (042930/z/94), and the West Midlands Perinatal Audit (CKA 313). DAS is a Research Fellow supported by the West Midlands Perinatal Audit.
References
- 1.Hackett GA, Campbell S, Gamsu H, Cohen-Overbeek T, Pearce JM: Doppler studies in the growth retarded fetus and prediction of neonatal necrotising enterocolitis, haemorrhage, and neonatal morbidity. Br Med J 1987, 294:13-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Macara L, Kingdom JC, Kaufmann P, Kohnen G, Hair JMI, Lyall F, Greer IA: Structural analysis of placental terminal villi from growth-restricted pregnancies with abnormal umbilical artery Doppler waveforms. Placenta 1996, 17:37-48 [DOI] [PubMed] [Google Scholar]
- 3.Jackson MR, Walsh AJ, Morrow RJ, Mullen JB, Lye SJ, Ritchie JW: Reduced placental villous tree elaboration in small-for-gestational-age pregnancies: relationship with umbilical artery Doppler waveforms. Am J Obstet Gynecol 1995, 172:518-525 [DOI] [PubMed] [Google Scholar]
- 4.Strain AJ, McGowan JA, Bucher NLR: Stimulation of DNA synthesis in primary cultures of adult rat hepatocytes by rat platelet-associated substance(s). In Vitro 1982, 18:108-116 [DOI] [PubMed] [Google Scholar]
- 5.Boros P, Miller MM: Hepatocyte growth factor: a multifunctional cytokine. Lancet 1995, 345:293-295 [DOI] [PubMed] [Google Scholar]
- 6.Matsumoto K, Nakamura T: Emerging multipotent aspects of hepatocyte growth factor. J Biochem 1996, 119:591-600 [DOI] [PubMed] [Google Scholar]
- 7.Zarnegar R, Michalopoulos GK: The many faces of hepatocyte growth factor: from hepatopoiesis to hematopoiesis. J Cell Biol 1995, 129:1177-1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miyazawa K, Shimomura T, Kitamura N: Activation of hepatocyte growth factor in the injured tissues is mediated by hepatocyte growth factor activator. J Biol Chem 1996, 271:3615-3618 [DOI] [PubMed] [Google Scholar]
- 9.Shimomura T, Kondo J, Ochiai M, Naka D, Miyazawa K, Morimoto Y, Kitamura N: Activation of the zymogen of hepatocyte growth factor activator by thrombin. J Biol Chem 1993, 268:22927-22932 [PubMed] [Google Scholar]
- 10.Bottaro DP, Rubin JS, Faletto DL, Chan AML, Kmiecik TE, Vande Woude GF, Aaronson SA: Identification of the hepatocyte growth factor receptor as the c-met proto-oncogene product. Science 1991, 251:802-804 [DOI] [PubMed] [Google Scholar]
- 11.Comoglio PM: Structure, biosynthesis, and biochemical properties of the HGF receptor in normal and malignant cells. Hepatocyte Growth Factor-Scatter Factor (HGF-SF) and the c-Met Receptor. Edited by ID Goldberg, EM Rosen. Birkhauser Verlag, Basel, pp 131–165
- 12.Sonnenberg E, Weidner KM, Birchmeier C: Expression of the met- receptor and its ligand, HGF-SF, during mouse embryogenesis. Hepatocyte Growth Factor-Scatter Factor (HGF-SF) and the c-Met Receptor. Edited by ID Goldberg, EM Rosen. Birkhauser Verlag, Basel, pp 381–394
- 13.Birchmeier C, Sonnenberg E, Weidner KM, Walter B: Tyrosine kinase receptors in the control of epithelial growth and morphogenesis during development. BioEssays 1993, 15:185-190 [DOI] [PubMed] [Google Scholar]
- 14.Kolatsi-Joannou M, Moore R, Winyard PJ, Woolf AS: Expression of hepatocyte growth factor/scatter factor and its receptor, MET, suggests roles in human embryonic organogenesis. Pediatr Res 1997, 41:657-665 [DOI] [PubMed] [Google Scholar]
- 15.Bladt F, Riethmacher D, Isenmann S, Aguzzi A, Birchmeier C: Essential role for the c-met receptor in the migration of myogeneic precursor cells into the limb bud. Nature 1995, 376:768-771 [DOI] [PubMed] [Google Scholar]
- 16.Schmidt C, Bladt F, Goedecke S, Brinkmann V, Zschieshe W, Sharpe M, Gherardi E, Birchmeier C: Scatter factor/hepatocyte growth factor is essential for liver development. Nature 1995, 373:699-702 [DOI] [PubMed] [Google Scholar]
- 17.Uehara Y, Minowa O, Mori C, Shiota K, Kuno J, Noda T, Kitamura N: Placental defect and embryonic lethality in mice lacking hepatocyte growth factor/scatter factor. Nature 1995, 373:702-705 [DOI] [PubMed] [Google Scholar]
- 18.Kilby MD, Afford S, Li XF, Strain AJ, Ahmed A, Whittle MJ: Localisation of hepatocyte growth factor and its receptor (c-met) protein and mRNA in human term placenta. Growth Factors 1996, 13:1-7 [DOI] [PubMed] [Google Scholar]
- 19.Clark DE, Smith SK, Sharkey AM, Sowter HM, Charnock-Jones DS: Hepatocyte growth factor/scatter factor and its receptor c-met: localisation and expression in the human placenta throughout pregnancy. J Endocrinol 1997, 151:459-467 [DOI] [PubMed] [Google Scholar]
- 20.Kauma S, Hayes N, Weatherford S: The differential expression of hepatocyte growth factor and met in human placenta. J Clin Endocrinol Metab 1997, 82:949-954 [DOI] [PubMed] [Google Scholar]
- 21.Chang TC, Robson SC, Spencer JA, Gallivan S: Identification of fetal growth retardation: comparison of Doppler waveform indices and serial ultrasound measurements of abdominal circumference and fetal weight. Obstet Gynecol 1993, 82:230-236 [PubMed] [Google Scholar]
- 22.Laemmli UK: Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227:680-685 [DOI] [PubMed] [Google Scholar]
- 23.Chomczynski P, Sacchi N: Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 1987, 162:156-159 [DOI] [PubMed] [Google Scholar]
- 24.Sangha RK, Li XF, Shams M, Ahmed A: Fibroblast growth factor receptor-1 is a critical component for endometrial remodeling: localization and expression of basic fibroblast growth factor and FGF-R1 in human endometrium during the menstrual cycle and decreased FGF-R1 expression in menorrhagia. Lab Invest 1997, 77:389-402 [PubMed] [Google Scholar]
- 25.Bussolino F, Di Renzo MF, Ziche M, Bocchietto E, Olivero M, Naldini L, Gaudino G, Tamagnone L, Coffer A, Comoglio PM: Hepatocyte growth factor is a potent angiogenic factor which stimulates endothelial cell motility and growth. J Cell Biol 1992, 119:629-641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saito S, Sakakura S, Enomoto M, Ichijo M, Matsumoto K, Nakamura T: Hepatocyte growth factor promotes the growth of cytotrophoblasts by the paracrine mechanism. J Biochem 1995, 117:671-676 [DOI] [PubMed] [Google Scholar]
- 27.Stoker M, Perryman M: An epithelial scatter factor released by embryo fibroblasts. J Cell Sci 1985, 77:209-223 [DOI] [PubMed] [Google Scholar]
- 28.Montesano R, Matsumoto K, Nakamura T, Orci L: Identification of a fibroblast-derived epithelial morphogen as hepatocyte growth factor. Cell 1991, 66:901-908 [DOI] [PubMed] [Google Scholar]
- 29.Grant DS, Kleinman HK, Goldberg ID, Bhargava MM, Nickoloff BJ, Kinsella JL, Polverini P, Rosen EM: Scatter factor induces blood vessel formation in vivo. Proc Natl Acad Sci USA 1993, 90:1937-1941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosen EM, Grant DS, Kleinman HK, Goldberg ID, Bhargava MM, Nickoloff BJ, Kinsella JL, Polverini P: Scatter factor (hepatocyte growth factor) is a potent angiogenesis factor in vivo. Symp Soc Exp Biol 1993, 47:227-234 [PubMed] [Google Scholar]
- 31.Ahmed A, Kilby MD: Hypoxia or hyperoxia in placental insufficiency? Lancet 1997, 350:826-827 [DOI] [PubMed] [Google Scholar]
- 32.Ahmed A: Heparin-binding angiogenic growth factors in pregnancy: a review. Trophoblast Res 1997, 10:215-258 [Google Scholar]
- 33.Jackson MR, Mayhew TM, Boyd PA: Quantitative description of the elaboration and maturation of villi from 10 weeks of gestation to term. Placenta 1992, 13:357-370 [DOI] [PubMed] [Google Scholar]
- 34.Kaufmann P, Luckhardt M, Leiser R: Three dimensional representation of the fetal vessel system in the human placenta. Trophoblast Res 1988, 3:113-137 [Google Scholar]
- 35.Rosen EM, Jaken S, Carley W, Luckett PM, Setter E, Bhargava M, Goldberg ID: Regulation of motility in bovine brain endothelial cells. J Cell Physiol 1991, 146:325-335 [DOI] [PubMed] [Google Scholar]
- 36.Somerset DA, Ahmed A, Kilby MD: The role of hepatocyte growth factor and its receptor, c-met, in placental development and fetal growth restriction: a review. Trophoblast Res 1998, 11:159-176 [Google Scholar]
- 37.Jin L, Pang Y-YS, Joseph A, Li Y, Rosen EM, Bhargava MM, Goldberg ID: Rat placental hepatocyte growth factor/scatter factor: purification, characterization, and developmental regulation. Proc Soc Exp Biol Med 1993, 204:75-80 [DOI] [PubMed] [Google Scholar]
- 38.Horibe N, Okamoto T, Itakura A, Nakanishi T, Suzuki T, Kazeto S, Tomoda Y: Levels of hepatocyte growth factor in maternal serum and amniotic fluid. Am J Obstet Gynecol 1995, 173:937-942 [DOI] [PubMed] [Google Scholar]