TABLE 2.
Changes in stability and unfolding kinetics for TII27, TNfn3, and their mutants in SDS and chemical denaturants
| Protein | ΔΔGD-N* |
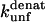 † †
|
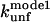 ‡ ‡
|
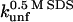 § §
|
|---|---|---|---|---|
| TNfn3 wild-type | ≡ 0 | 7.21 ± 0.26 × 10−5 | 5.81 × 10−3 | 1.3 × 10−2 |
| TN P25A | 1.73 ± 0.13 | 1.92 ± 0.46 × 10−3 | 1.02 × 10−1 | 1.04 |
| TN I48A | 2.19 ± 0.12 | 2.32 ± 0.33 × 10−4 | 9.3 × 10−3 | 8.09 × 10−2 |
| TN Y57G | 4.14 ± 0.10 | 1.29 ± 0.22 × 10−2 | 4.12 × 10−1 | 3.32 |
| TN L62A | 4.22 ± 0.11 | 7.91 ± 1.40 × 10−3 | 8.39 × 10−2 | 1.57 × 10−1 |
| TN T66A | 1.96 ± 0.12 | 8.11 ± 1.80 × 10−4 | 3.2 × 10−2 | 9.41 × 10−2 |
| TII27 wild-type | ≡ 0 | 4.9 ± 0.6 × 10−4 | 3.22 ± 2.1 × 10−4 | 3.17 ± 0.23 × 10−3 |
| TI V4A | 2.45 ± 0.10 | 1.3 ± 0.1 × 10−3 | 6.98 × 10−3 | 2.07 × 10−2 |
| TI L8A | 2.45 ± 0.10 | 1.2 ± 0.2 × 10−3 | 1.54 ± 0.54 × 10−2 | 3.72 ± 0.89 × 10−2 |
| TI L36A | 3.3 ± 0.10 | 3.9 ± 0.3 × 10−3 | 1.96 ± 0.19 × 10−2 | 1.31 ± 0.00 × 10−1 |
| TI L60A | 4.88 ± 0.08 | 3.4 ± 0.3 × 10−3 | 1.87 ± 0.22 × 10−2 | 3.26 ± 0.37 × 10−2 |
The mutation-induced change in the difference in free energy between the denatured state, D, and the native state, N, on mutation (kcal·mol−1), relative to wild-type protein. Obtained from Fowler and Clarke (15) (TII27) and Hamill et al. (43) (TNfn3).
The 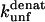 (s−1) values are obtained from Fowler and Clarke (15) (TII27, GdmCl) and Hamill et al. (43) (TNfn3, Urea). For TII27 the authors determined
(s−1) values are obtained from Fowler and Clarke (15) (TII27, GdmCl) and Hamill et al. (43) (TNfn3, Urea). For TII27 the authors determined 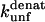 by linear extrapolation of log
by linear extrapolation of log 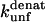 at different denaturant concentrations to zero molar denaturant;
at different denaturant concentrations to zero molar denaturant; 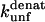 for TNfn3 mutants was calculated from Hamill et al. (43) by use of the following equation: Δ
for TNfn3 mutants was calculated from Hamill et al. (43) by use of the following equation: Δ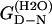 (mutant) = −RTln(ku/kf)mutant.
(mutant) = −RTln(ku/kf)mutant.
Unfolding rate constant (s−1) in SDS at the plateau level (TII27) and at the highest level before inhibition sets in (TNfn3) (cfr. Figs. 7 and 8). Errors are from the direct fits to Eq. 2 (TNfn3) or Eq. 3 (TII27). In several cases, it was not possible to obtain a stable fit, making it necessary to estimate the value by visual inspection.
Unfolding rate constant (s−1) interpolated to 500 mM SDS.
