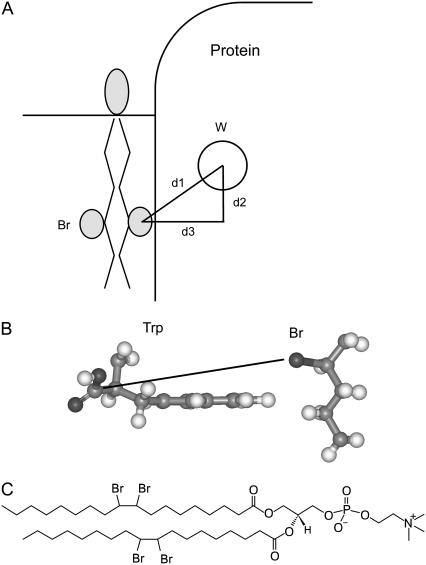
FIGURE 5.
Modeling fluorescence quenching in MscL. (A) The efficiency of quenching of the Trp fluorescence in a membrane protein by a neighboring lipid molecule with bromine-containing fatty acyl chains depends on the distance d1 between them. The distance d2 can be calculated from the position of the Trp residue in the protein and the location of the bromines in the bilayer. The distance d3 depends on the depth of the Trp residue below the protein surface and the extent of penetration of the lipid fatty acyl chains into the surface, as described in the text. (B) The figure shows a Trp residue and a bromine-containing alkane with van der Waals contact between the edge of the Trp ring and the alkyl chain. The Trp α-carbon-to-Br distance marked is 9.4 Å. (C) Structure of di(Br2C18:0)PC.