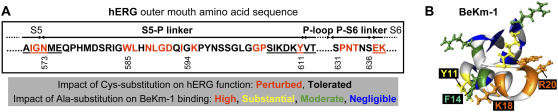
FIGURE 1.
(A) Amino acid sequence lining the outer vestibule of hERG: residues 573–611 (S5-P linker), and residues 631–636 (P-S6 linker). Segment 585–594 corresponds to a helical region determined by previous NMR studies (15,16). Red lettering highlights high-impact positions based on a previous cysteine scanning mutagenesis study (14). (B) Three-dimensional structure of BeKm-1 in ribbon format, with positions color coded for effects of alanine substitution on toxin binding to hERG: high (brown), substantial (yellow), moderate (green), and negligible (blue). BeKm-1 positions not tested are color coded light gray. Side chains for the first three classes of position are shown in ball-and-stick format. Side chains of Y11, F14, K18, and R20 are labeled.