Figure 3.
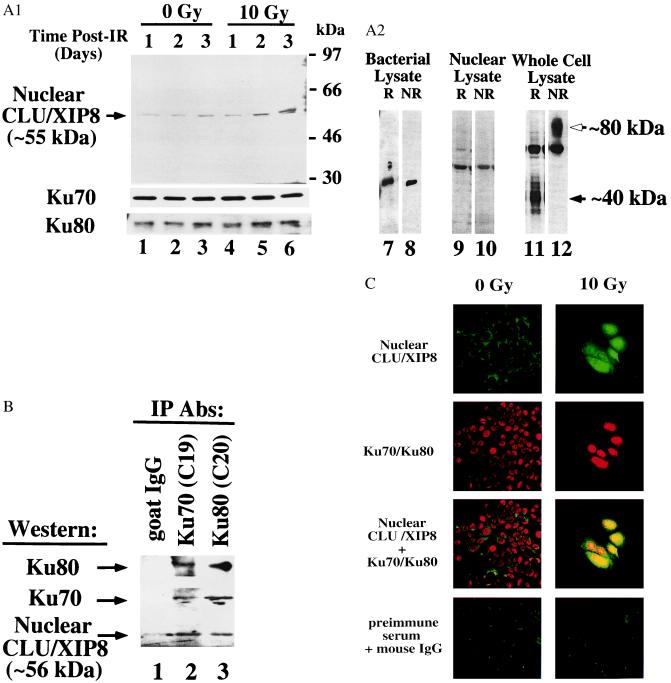
Analyses of Ku70, Ku80, and CLU/XIP8 in nuclear lysates of MCF-7 cells after IR. (A) Log-phase MCF-7 cells were treated with or without 10 Gy and nuclear lysates prepared at various times (lanes 1–6). Ku70 and Ku80 levels served as loading controls. The 55-kDa form of nuclear CLU/XIP8, Ku70, and Ku80 were detected by Western blot analyses using anti-CLU/XIP8 (rCY1), Ku70 (N3H10), or Ku80 (LL1) antibodies, respectively. CLU/XIP8 produced in bacteria (lanes 7 and 8) or found in IR-exposed MCF-7 nuclei (lanes 9 and 10) was not altered under reducing (R, with β-mercaptoethanol) or nonreducing (NR, without β-mercaptoethanol) conditions. In contrast, secretory CLU/XIP8 protein, detected by the 41D antibody, was converted from its native 80-kDa form (open arrow) to 40-kDa α and β glycopeptides (closed arrow) as indicated (lanes 11 and 12). (B) Nuclear lysates prepared from MCF-7 cells 3 days after 10 Gy were immunoprecipitated by using Ku70 (C19) or Ku80 (C20) antibodies. Coimmunoprecipitates were detected by Western blotting using anti-CLU/XIP8 (rCY1), Ku70 (N3H10), or Ku80 (LL1) antibodies. Goat IgG was used as a negative control. (C) Colocalization of CLU/XIP8 with Ku70/Ku80 in the nuclei of MCF-7 cells treated with IR by confocal microscopy. MCF-7 cells were treated with or without IR (10 Gy) and fixed 3 days post-IR. Cells were doubly stained by using rabbit anti-CLU/XIP8 (rCY1) and mouse anti-Ku70/Ku80 heterodimer (162) antibodies. Secondary antibodies were FITC-anti-rabbit (green) and Texas red-anti-mouse. Colocalization of CLU/XIP8 (green) and Ku70/Ku80 (red) was shown by yellow in “giant” irradiated cells. Untreated MCF-7 cells showed no colocalization of proteins. Digital images were taken at ×400 magnification. Preimmune serum and normal mouse IgG were used as negative controls. Experiments in A–C were repeated three or more times.