Fig. 1.
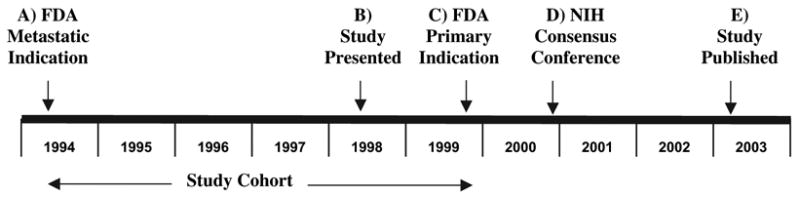
Timeline of major events around the approval of paclitaxel for primary breast cancer. The U.S. Food and Drug Administration (FDA) approved the use of paclitaxel for metastatic breast cancer in April 1994 (A). Results from Cancer and Leukemia Group B (CALGB) Study 9344 were presented at the American Society of Clinical Oncology Annual Meeting in May 1998 (B). FDA approved paclitaxel for lymph node–positive primary breast cancer I October 1999 (C). In November 2000, a National Institutes of Health Consensus Conference stated that “Currently available data regarding the use of taxanes in the adjuvant treatment of women with node-positive breast cancer are inconclusive, ” (21) (D). The CALGB study was published in March 2003 (E). The position of the study cohort relative to these events is shown below the timeline.
