Abstract
Impaired lipoprotein metabolism is one of the major aetiological factors for the pathogenesis of atherosclerosis and cardiovascular disease (CVD). Assessment is usually made in the fasting state, and particular attention is directed towards the measurement of the cholesterol content of both the low and high-density lipoprotein fractions. By comparison, a massive amount of lipid fluxes through the intra-vascular compartment during the post-prandial period. This has led to the hypothesis that atherosclerosis could be partially, or even predominantly, due to the pathological effects of this flux of post-prandial lipoproteins on the vessel wall. This justifies efforts to systematically study the relationship between the lipoprotein responses to food (particularly fat) ingestion and cardiovascular disease or its surrogate markers. This review will consider the mechanisms by which post-prandial metabolism might affect the risk of CVD. It will examine the evidence for and against such an association. It will also consider the practical and methodological issues that are likely to determine the future utility of post-prandial lipoprotein assessment.
Introduction
Impaired lipoprotein metabolism is now well established as one of the major treatable risk factors for CVD. It is usually assessed in terms of the fasting level of atherogenic low-density lipoprotein (LDL) cholesterol and anti-atherogenic high-density lipoprotein (HDL) cholesterol. Measurements are usually made in the fasting state for several reasons, including facilitation of the calculation of the level of LDL-cholesterol.1 The fasting state is not representative of the metabolic circumstances that apply to people most of the time. Morton’s early observations, together with the work of Fraser and Zilversmit, led to the “post-prandial theory of atherosclerosis” as an alternative or additional explanation for the aetiology of CVD.2–4 This theory suggests that the development of atherosclerosis is a more episodic process that largely depends on the metabolic response to individual meals. One limitation of the theory is that it only considers post-prandial metabolism in pro-atherogenic terms. As in the case of the fasting state, it is more plausible that post-prandial lipid metabolism creates a range of pro- and anti-atherosclerotic phenomena that may or may not cause a net contribution to CVD, depending on a variety of circumstances. It is important to understand these phenomena in some detail.
This review examines the practical aspects of post-prandial assessment, and the current picture it provides of lipid metabolism. It considers the patho-physiological effects that the post-prandial state may exert on the artery wall in terms of atherosclerosis and its prevention. Other aspects of CVD such as thrombogenesis and vascular reactivity are also discussed. Interaction with other macronutrients is considered with particular emphasis on carbohydrate metabolism and insulin resistance. Clinical trial results are examined for evidence of an independent effect by post-prandial lipoproteins. The review concludes with reflections about the prospects for investigations and interventions that specifically target the post-prandial state.
Clinical Assessment
Comparison with Fasting Measurements
The lifestyle determinants of the post-prandial lipid response differ from those for fasting triglyceride (TG),5 but non-fasting TG is similar to fasting TG in its predictive value for CVD.6 Nevertheless, fasting TG level remains one of the main determinants of post-prandial lipid metabolism.7 Unfortunately, there are several issues that have frustrated attempts to increase our understanding of the relationship between post-prandial metabolism and CVD. Procedures for post-prandial testing have yet to be standardised. They are inherently more time-consuming and labour-intensive than fasting investigations and it is more difficult to condense post-prandial data into a form that is easily interpreted. These limitations make it difficult to directly compare the conclusions of various clinical studies. As a result, there is a relative paucity of data.
Nevertheless, there is enough preliminary information to suggest that post-prandial lipid metabolism can strongly influence the risk of CVD. The lipoprotein changes that accompany the insulin resistance syndrome have highlighted the importance of TG-rich lipoproteins (TRL), that include both chylomicrons and very low-density lipoproteins (VLDL) and their remnants.8 The impact of these metabolic changes is likely to be amplified following fat ingestion. Attempts to integrate the assessment of the metabolism of the major macronutrients (carbohydrates and lipids) by examining their inter-relationship after the ingestion of a mixed meal is still at an early stage.9 Although this will require more complex assessment, it is important to reflect the homeostatic interdependence of macronutrient metabolism because it involves integration by regulatory hormones such as insulin.10
Lipoproteins
Traditional methods for the separation of lipoproteins are not capable of completely isolating particles of intestinal origin from those synthesised in the liver.11 In particular, the remnants formed by the partial catabolism of intestinal chylomicrons following hydrolysis by lipoprotein lipase (LPL) cannot be separated from those formed by the similar hydrolysis of VLDL of hepatic origin.12 The similarity between the remnants from these two sources in terms of density and electrophoretic mobility prevents resolution by ultracentrifugation or electrophoresis, whilst their overlap in size prevents separation by new techniques such as size-exclusion chromatography. Newly secreted chylomicrons can be separated and sub-classified by ultracentrifugation.13 However, it is probably the smaller remnant fraction that is more relevant to pathological effects on the vessel wall. This inability to isolate the components of the spectrum of intestinally derived lipoproteins poses a substantial obstacle to the study of post-prandial lipoprotein metabolism.
A well-established technique for the study of intestinally-derived lipoproteins involves the oral administration of retinol. This is esterified during intestinal absorption and then transported in the core of chylomicrons, mainly as retinyl palmitate.14 Hepatic uptake of the chylomicron remnants results in conversion to retinol, which only re-enters the circulation in association with retinol-binding protein. Theoretically, the level of retinyl ester following retinol administration should be proportional to the level of core lipids in chylomicrons and their remnants. Some studies suggest that there could be substantial exchange of retinyl esters between intestinal and non-intestinal lipoproteins,15 whilst others imply that intestinal remnants account for all the retinyl ester in d<1.063 g/mL fractions.16 In either case, the usefulness of this marker is likely to be superseded by newer methods.
Intestinally-derived TRL have a distinguishing apolipoprotein (apo) in the form of apoB48. This is a truncated equivalent to hepatically-synthesised apoB100. ApoB48 is formed by the action of the apoB-editing enzyme (APOBEC) in enterocytes which converts a nucleotide base in apoB mRNA to produce a stop codon.17 There is one molecule of the truncated apoB48 per intestinal TRL particle.18 ApoB48 lacks the LDL receptor-binding domain, and although its sequence is identical to the amino terminus of apoB100 molecule, apoB48 may be distinguished from other apolipoproteins, including apoB100, by immunoassay19 or polyacrylamide gel electrophoresis (PAGE).20 The rate of apoB48 secretion by the intestine is relatively constant, whilst the degree of lipid enrichment and the rate of catabolism are more variable.21 This implies that enterocytes control intestinal lipid transport by modifying the composition of the secreted lipoproteins, rather than their absolute number.
The clinical measurement of apoB48 has been limited by the complexity and lack of standardisation of the available assays. The PAGE methods utilise specialised equipment and require the preparation of a purified apoB48 standard, whilst immuno-assay methods require validation of the ability of the anti-apoB48 antibody to distinguish the conformational change in the apoB moiety of all intestinally-derived lipoproteins in the presence of those of hepatic origin. As expected, the limited studies conducted so far reveal very low fasting levels of apoB48 that rise substantially after fat ingestion.22 Pathological states in which TG is elevated are associated with an increase in fasting and post-prandial apoB48 levels.8 Furthermore, higher post-prandial and fasting levels of apoB48 have been reported in patients with coronary heart disease (CHD)23 and diabetes,24 but apoB48 is yet to be established as an independent predictor of CVD.
An immuno-affinity method exploits differences in the apolipoprotein composition of TRL to remove those of hepatic origin that contain apoB100.25 After apoA-I removal, the remaining fraction consists of apoB48-containing chylomicrons and their remnants, together with an apoE-rich VLDL remnant fraction in which the apoB100 epitope has been masked. The measurement of the cholesterol and TG levels in this remnant fraction may provide insight into the effect of these lipoproteins on the risk of CVD but the relative contributions from intestinal and non-intestinal particles cannot be differentiated. Nevertheless, increased post-prandial remnant cholesterol levels were associated with endothelial dysfunction,26 and fasting levels measured with this method were predictive of future CVD events.27 ApoC-III, which is another component of remnants and their precursors, is also associated with the risk of CVD. However, the contribution of intestinal and non-intestinal particles is once again unclear.
Clinical Investigations
The apparent superiority of impaired glucose tolerance over impaired fasting glucose as a predictor of future macrovascular disease suggests that an equivalent loading test to increase the sensitivity of lipoprotein measurements could improve prediction of future CVD.28 There are several reasons why an equivalent widely accepted lipid-loading test has not been developed so far. Firstly, the absorption of a lipid load is less predictable due to variability of gastric emptying. Interventions that alter gastric emptying have been shown to have significant effects on acute and chronic responses of TG and glucose ingestion.29 Although the problem can be by-passed by administration of intravenous lipid material, this approach fails to assess the intestinal synthetic component of the process. Other solutions, such as the administration of drugs to stimulate gastric emptying, or duodenal cannulation techniques, would complicate interpretation and compromise the widespread use of such tests. Secondly, the question of “What to administer?” poses a more substantial problem in the case of lipids. An oral load of unadulterated lipid would be a challenge in terms of palatability, whilst the addition of other macronutrients would alter associated regulatory responses such as insulin secretion.9 The composition of the lipid load could also vary in terms of both fatty acid composition and the presence or absence of other lipids, especially cholesterol.30 The amount to be administered is another source of variability. Should an absolute amount be administered, or should a dose be calculated on a patient-specific basis such as body surface area? Should the load be designed to result in an exaggerated response, or should it attempt to mimic average levels of intake? The number and timing of measurements is another aspect that currently lacks standardisation. Control of the preceding diet has been shown to reduce the intra-individual variability of the post-prandial response,31 as has avoidance of exercise following administration of the fat load. None of these aspects have been standardised, and in the absence of a more compelling case for post-prandial lipid testing, it seems doubtful that they will be in the near future.
Laboratory assessment is another area in which studies vary. The prolonged response to dietary fat intake requires sustained monitoring, but the exact timing of sample collection has remained somewhat arbitrary. Most studies emphasise time points between two and eight hours, but evidence suggests that the main independent predictors of CVD occur considerably later.32,33 TG levels nine hours after an oral fat load are more reproducible than fasting levels, suggesting that later time-points should be selected.34 The selection of analyses is another area of variation. The level and pattern of post-prandial TG levels has received the most emphasis, but this is highly correlated with the fasting TG level. The time-course of changes in the composition of TRL may reflect alterations in cholesterol content of this fraction, and this may be due, at least in part, to reciprocal changes in the HDL fraction.35 Measurement of apoB48 or retinyl palmitate provides a means of attempting to relate these changes to alterations in the proportion of intestinally-derived particles. In animal experiments, intestinally-derived particles may be labelled and their recovery from plasma and tissues may be assessed. The opportunities for similar studies in humans are limited, but surrogate markers such as the production of carbon dioxide from lipid labelled with stable isotope can provide an index of lipid uptake and oxidation.36
The volume and variety of measurements obtained during post-prandial studies present a problem in presentation of data. How should such large amounts of information be condensed into succinct results that convey the essential features of an individual’s response to lipid ingestion? Many studies use the trapezoid rule to present the area under the curve for various analytes, but these are often closely correlated with fasting TG, even after normalisation for the baseline level.32 Even if a summary statistic could be established, there is insufficient data at present to establish a relationship with clinical endpoints in order to select a cut-off for medical decision-making.
Despite these problems of clinical evaluation, there is abundant evidence to suggest that the metabolic events that accompany ingestion of lipids are extremely important in the pathogenesis of CVD.35,37 Examination of this evidence may assist the formulation of recommendations for standardisation of post-prandial testing.
Lipoprotein Metabolism
Chylomicrons
Post-prandial metabolism might affect atherosclerosis and the risk of CVD via a number of different mechanisms. Firstly, the intestinally-derived lipoprotein particles might directly affect atherosclerosis or other aspects of CVD. Secondly, the post-prandial flux of lipid through the vascular compartment might modify the metabolism of non-intestinal lipoproteins or other metabolites in a fashion that affects their atherogenicity. Finally, post-prandial metabolism may directly affect other processes that affect CVD such as endothelial function and thrombosis.
The clinical features of hereditary LPL deficiency were thought to suggest that chylomicrons did not contribute to atherosclerosis unless they underwent lipolysis, consistent with the concept that uncatabolised chylomicrons were too large to penetrate the endothelium. However, this theory has now been called into question.38,39 The issue of whether or not chylomicron remnants can directly contribute to atherosclerosis or not remains complicated, but evidence tends to favour the possibility that they may. There is plentiful evidence that TRL remnants can contribute to atherosclerosis in animals 40 and humans.41 The clinical example of CVD following remnant accumulation due to ApoE2 homozygosity,42 and atheroma in animal models following apoE gene knockout 43 suggest that these remnants are particularly atherogenic. Furthermore, animal evidence suggests that intestinally-derived lipoproteins can contribute to this process.44 Whilst the demonstration of apoB48 in human plaque has been problematical,45 largely because of methodological limitations, it has also been postulated that surface components of remnant lipoproteins may exert an atherogenic effect due to their cytotoxicity.46
Even if intestinally-derived remnant lipoproteins do not directly contribute to human plaque, it is likely that these chylomicron remnants could exert several indirect actions on atherosclerosis. Nascent chylomicrons containing apoB48 and ApoA-I, II and IV acquire ApoE and ApoC-II and III when they enter the vascular compartment. The apoC’s control hydrolysis by endothelial LPL which transforms the chylomicron into a smaller remnant. The composition of the chylomicron and its remnant is also likely to be affected by the action of cholesteryl ester transfer protein (CETP) which allows the exchange of cholesteryl ester and TG between lipoprotein classes. Chylomicron remnants are rapidly removed from the circulation in the liver, but this requires sequestration into the Space of Disse via endothelial fenestrations in the hepatic sinusoids.47 Loss of fenestrations with age 48 and reduced hepatic clearance of remnants have been reported in the elderly.49 Sequestration within the Space of Disse is further augmented by apolipoproteins, proteoglycans, hepatic and endothelial lipases, which assist molecular interaction between remnant lipoproteins and specific receptors. Complete removal involves receptor-mediated endocytosis, particularly by the LDL receptor; however, the LDL receptor-related protein (LRP) also appears to play an ancillary role.50 The involvement of the LDL receptor in the clearance of chylomicron remnants infers a mechanism by which these remnants might affect CVD. Removal of chylomicron remnants via this pathway competitively reduces the clearance of LDL, and this may exert a non-acute cumulative effect leading to an increase in plasma LDL levels.51
The action of CETP not only increases the cholesterol content of chylomicron remnants, but also modifies the composition of non-intestinally derived lipoproteins. This may be seen as a transient decrease in the level of HDL-cholesterol during the post-prandial phase.35 The action of CETP could also modify the composition of LDL particles, making them smaller and denser. Excessive post-prandial CETP activity driven by exaggerated post-prandial hypertriglyceridaemia may therefore increase the risk of CVD via several mechanisms. Firstly, it may increase the atherogenicity of chylomicron remnants by cholesterol ester enrichment. Secondly, it may reduce the protective effect of HDL by lowering the level of HDL cholesterol. Finally, it may increase the atherogenicity of LDL by increasing the proportion of small dense LDL particles.
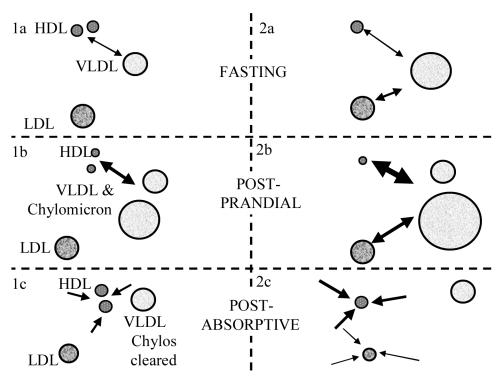
The size of LDL does not seem to be acutely affected during the post-prandial response. On the other hand, there is evidence to suggest a cumulative effect. Our studies identified the level of post-prandial TRL as the strongest determinant of LDL particle size 52 and this association has also been reported elsewhere.53,54 A possible explanation is illustrated in Figure 1. Post-prandial enhancement of cholesterol ester transfer is likely to remove cholesterol ester from LDL as well as HDL, especially when HDL-cholesterol has become relatively depleted. The cholesterol deficit in HDL can be substantially replaced during the post-absorptive period by transfer of free cholesterol followed by esterification by lecithin: cholesterol acyltransferase (LCAT). However, the post-prandial removal of cholesterol ester from LDL cannot be replaced to the same extent because LCAT esterification associated with LDL is much less active. As a result, sequential episodes of exaggerated post-prandial TRL accumulation are likely to lead to a cumulative reduction in LDL particle size.
Figure 1.
Hypothetical explanation for the association between excessive post-prandial lipaemia and LDL particle size. Panel 1: Normal cholesterol ester transfer protein activity exchanges HDL-cholesterol for VLDL triglyceride in the fasting state (1a). This exchange is enhanced due to the presence of increased triglyceride in the form of chylomicrons during post-prandial lipaemia (1b). The HDL can be replenished by free cholesterol transfer followed by esterification once chylomicrons have been cleared in the post-absorptive phase (1c). Panel 2: Recurrent episodes of exaggerated post-prandial lipaemia deplete fasting HDL-cholesterol levels (2a). Further episodes of exaggerated post-prandial lipaemia require transfer of LDL-cholesterol to compensate for the deficit (2b). Whilst HDL-cholesterol can be partly replenished as previously described, LDL-cholesterol depletion is more permanent. The relative lack of LCAT activity associated with LDL means that even if free cholesterol transfers to LDL, esterification will be far more limited, so levels will remain low (2c).
On the other hand, the post-prandial actions of CETP may not necessarily be pro-atherogenic. Modest post-prandial lipaemia may enhance reverse cholesterol transport (RCT). In vitro evidence suggests that post-prandial TRL may enhance cholesterol efflux under some circumstances55whilst in vivo studies indicate that HDL phospholipid increases post-prandially.56,57 The net effect is likely to depend on the complex interaction of a number of factors including plasma lipoprotein levels, intravascular metabolic processes and the capacity for hepatic uptake of remnants. Impairment of the process has been noted in patients with coronary artery disease (CAD).58 Post-prandial RCT may be more severely impaired in insulin resistance and type 2 diabetes.59
Post-prandial events also affect the metabolism of lipoprotein (a) [Lp(a)]. The presence of chylomicrons causes Lp(a) to form a non-covalent association with TRL with the result that a significant proportion of Lp(a) can be isolated from the d < 1.006 g/mL fraction of post-prandial plasma.60 The extent of association is proportional to the molecular weight of the apolipoprotein (a) isoforms,61 and the phenomenon reverses as the post-prandial phase resolves.62 It is important to note that complex formation is more pronounced with the higher molecular weight isoforms that are associated with a lower risk of CVD.63 The significance of this process is yet to be determined. It may modify the propensity for TRL to cause accumulation of lipid within macrophages.64 Alternatively, the interaction between Lp(a) and TRL may modulate the role of sinusoidal fenestrations in the hepatic clearance of chylomicron remnants via the Space of Disse.47 The TRL-Lp(a) complex has a greater diameter than un-complexed chylomicrons and their remnants, and as a result, the complexes are more likely to remain in circulation. The complex dissociates after sufficient lipolysis has taken place, after which the chylomicron remnant would be more likely to pass through the sinusoidal fenestration into the space of Disse. Furthermore, Lp(a) secreted into the Space of Disse may retain chylomicrons and their remnants by forming a complex that prevents their escape. It is also possible that this interaction may affect the way in which chylomicron remnants interact with proteoglycans, ApoE and lipases that are present in the space of Disse as facilitators of receptor-mediated uptake.65 The Lp(a) component of the complex may even modulate receptor-mediated uptake processes because it is likely to reduce interaction with LDL receptors 66 whilst enhancing interaction with LRP.67
This uncertainty about the implications of CETP activity and Lp(a) metabolism during the post-prandial phase suggests that it is over-simplistic to regard the post-prandial state as necessarily pro-atherogenic. Like the fasting state, it is likely that there is an inter-play between pro-atherogenic and anti-atherogenic aspects of lipoprotein metabolism. This poses the broader challenge of recognising both the harmful and the protective components in order to correct the former and enhance the latter.
Vessel Wall Response
A number of techniques, such as arterial compliance and assessment of vascular endothelial function by flow mediated vascular reactivity (FMV), have been established as surrogate markers for the risk of CVD. Several studies employing such techniques suggest that vascular function can be markedly affected in the post-prandial period.68–72Both hypertriglyceridaemia and hyperglycaemia have been associated with post-prandial impairment of endothelial function.73 As endothelial dysfuction appears to be a key early event in atherogenesis, this observation lends support to the concept that episodic meal-related events may have a cumulative effect on CVD.
Early studies suggested that fat ingestion was associated with impairment of the vascular endothelial response, and that this could be counteracted by administration of anti-oxidants or replacement of dietary fat by carbohydrate.67,74 The impairment correlated with the timing and degree of post-prandial hypertriglyceridaemia75 and, in some instances, markers of oxidative stress.76 The detrimental effects of a fatty meal may depend on qualitative rather than quantitative aspects of fat consumption because fatty acid composition or chemical changes induced by repeated use of cooking oil account for deterioration in vascular endothelial function in some studies.69 These findings have been supported by studies that used alternative methods of assessment of endothelial function, but some negative studies have also been reported.74,77–79 One of these studies showed an association between the level of remnant lipids and impaired baseline endothelial function, but fat ingestion did not cause further deterioration.77 Studies of the post-prandial response of resistance vessels have also shown some neutral results.80
Care is required in interpretation of these results because it is likely that the insulin response to any carbohydrate component of the test meals would have an independent effect. Insulin is well recognised as a potent vasodilator. Carbohydrate-containing lipid loads are likely to cause a confounding change in underlying vessel diameter.81 This makes it difficult to design an appropriate control meal that is equivalent, not only in terms of calories, but also in its ability to stimulate insulin release. It is also difficult to interpret the significance of percentage reductions in response if the baseline diameter of the vessel has altered in response to insulin. Furthermore, the vasodilatory response to insulin may diminish the residual capacity for further endothelium-dependent vasodilatation. Resolution of these issues will require reporting of absolute changes in all relevant post-prandial vascular parameters, rather than just the change in percentage dilatation. It is therefore interesting to note that intimal medial thickness, which is not dependent on intercurrent vasodilitation, is also impaired in subjects with increased post-prandial lipaemia.82
Post-prandial insulin-mediated vasodilitation may in fact represent a vascular-specific aspect of insulin action. It has been postulated that resistance to insulin-stimulated vasodilatation of adipose tissue and muscle may account for the failure to extract macronutrients in insulin resistance.83 It remains to be determined whether or not vascular resistance to insulin action represents a broader indication of the vessel’s vulnerability to the other consequences of insulin resistance, including susceptibility to macrovascular disease. The post-prandial lipid response also modulates insulin secretion84 and this response also appears to be altered in insulin resistance.85 Finally, post-prandial studies are also complicated by the need to differentiate the degree to which post-prandial hyperglycaemia (rather than post-prandial lipaemia) may be responsible for vascular impairment.86
Endothelial function may also be affected by other nutrients in the post-prandial phase. Marked impairment has been reported after intake of methionine. The severity of the impairment correlated with plasma homocysteine level, and the problem was eliminated by administration of sufficient folic acid to avoid hyperhomocysteinaemia.87 Folate also prevented deterioration in endothelial function induced by fat consumption.88 Conversely, large intakes of arginine acutely increase the availability of substrate for nitric oxide synthesis via endothelial nitric oxide synthase (eNOS). Reversal of impaired endothelial function has been demonstrated with arginine supplementation,89,90 but the effect may be short-lived.91
Athero-thrombosis
Post-prandial events may also affect the coagulation component of the athero-thrombotic process that underlies CVD events. There is a positive relationship between fasting TG and post-prandial lipaemia on one hand, and pro-thrombotic factors such as activated factor VII and plasminogen activator inhibitor-1 (PAI-1) on the other.92–95 This may be partially due to the fact that the phospholipids and other components of the coat of TRL mimic the surfaces that initiate the intrinsic coagulation pathway. Studies in patients with CAD also show that levels of activated factor VII increase during the chylomicronaemia associated with fat consumption, and it has been postulated that these haemostatic changes may trigger episodes of acute coronary syndrome.96
Clinical End-points
Results
It is clear from the evidence presented so far that important processes that may promote or retard CVD take place during the post-prandial period. Although several practical problems have been highlighted, clinical post-prandial assessment is feasible, but the question is whether or not the effort is justified. Post-prandial parameters have been associated with all forms of CVD.32,33,97–104 These cross-sectional case control studies may be susceptible to confounding factors such as the prevalence of beta-blocker use. On the other hand, the association is also predictive of progression of CVD,105 and it has been noted amongst close relatives of CVD patients.106 The most popular parameters include area under the TG and retinyl ester curves, but these are strongly correlated with fasting TG and tend to reflect the early phase of lipid absorption. The crucial issue is whether or not any post-prandial measurement provides additional information beyond that supplied by fasting levels, especially fasting TG. Several studies suggest that they do, because they displace fasting TG as an independent variable in multivariate analysis. However, this may largely be a reflection of reduced biological variability. More detailed analyses suggest that later time-points are more predictive 33 and that cholesterol content 32 or numbers of remnant lipoproteins 8 are more likely to be independent of fasting TG. Recent studies argue that fasting levels of apoB48 or remnant lipoprotein cholesterol may suffice for clinical assessment of CVD risk related to some aspects of post-prandial lipid metabolism.107–109
Conclusions
The issue of post-prandial assessment of metabolism is clinically important. The additional complexity associated with post-prandial testing will be justified if it provides a reliable means for quantifying CVD risk that is not detected by other means. Although disturbances in post-prandial lipid and carbohydrate metabolism may have some separate effects on the pathogenesis of CVD, it seems important to recognise the inter-dependence of these two components of macronutrient metabolism. This is particularly important in light of the increasing prevalence of obesity and insulin resistance, and it argues in favour of the establishment of a standardised test using a mixed meal. This is just one of the areas in which standardisation is required. The available data suggests that protocols should concentrate on late time-points (over eight hours after fat intake) and it may even be possible to use the fasting levels of some post-prandial markers such as apoB48 or remnant lipoprotein cholesterol.
The insights gained from the study of post-prandial metabolism may also identify new forms of intervention that act via modification of the intestinal absorption of fat and other nutrients. For example, it may detect differences caused by changes in meal size and frequency, or changes arising from lifestyle factors such as those associated with shift-work. It may identify the need for strategies that modify gastric emptying time or meal composition. This type of information is likely to be increasingly important due to the introduction of new lipid–lowering drugs such as ezetimibe, that act at the level of nutrient absorption. It may even be possible to manipulate some of the favourable components of the post-prandial state, such as increased cholesterol ester transfer, to obtain a clinical benefit in some instances. Most importantly, emphasis of the post-prandial component of lipid metabolism reinforces the fundamental link between dietary factors and the aetiology of CVD.
References
- 1.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low density lipoprotein cholesterol without the use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 2.Moreton JR. Atherosclerosis and dietary hyperlipemia. Science. 1947;106:190–1. doi: 10.1126/science.106.2748.190. [DOI] [PubMed] [Google Scholar]
- 3.Fraser R. The role of dietary triglycerides in cholesterol metabolism. Atherosclerosis. 1974;19:327–36. doi: 10.1016/0021-9150(74)90067-7. [DOI] [PubMed] [Google Scholar]
- 4.Zilmersmit DB. Atherogenesis: a post-prandial phenomenon. Circulation. 1979;60:473–85. doi: 10.1161/01.cir.60.3.473. [DOI] [PubMed] [Google Scholar]
- 5.Patsch W. Metabolic and lifestyle determinants of postprandial lipemia differ from those of fasting triglycerides: The Atherosclerosis Risk In Communities (ARIC) study. Arterioscler Thromb Vasc Biol. 2001;21:275–81. doi: 10.1161/01.atv.21.2.275. [DOI] [PubMed] [Google Scholar]
- 6.Eberly LE, Stamler J, Neaton JD. Multiple Risk Factor Intervention Trial Research Group. Relation of triglyceride levels, fasting and nonfasting, to fatal and nonfatal coronary heart disease. Arch Intern Med. 2003;163:1077–83. doi: 10.1001/archinte.163.9.1077. [DOI] [PubMed] [Google Scholar]
- 7.Miller M, Zhan M, Georgopoulos A. Effect of desirable fasting triglycerides on the postprandial response to dietary fat. J Investig Med. 2003;51:50–5. doi: 10.2310/6650.2003.33544. [DOI] [PubMed] [Google Scholar]
- 8.Mero N, Malmstrom R, Steiner G, Taskinen MR, Syvanne M. Post-prandial metabolism of apolipoprotein B-48- and B-100-containing particles in type 2 diabetes mellitus: relations to angiographically verified severity of coronary artery disease. Atherosclerosis. 2000;150:167–77. doi: 10.1016/s0021-9150(99)00364-0. [DOI] [PubMed] [Google Scholar]
- 9.Kriketos AD, Sam W, Schubert T, Maclean E, Campbell LV. Post-prandial triglycerides in response to high fat: role of dietary carbohydrate. Eur J Clin Invest. 2003;33:383–9. doi: 10.1046/j.1365-2362.2003.01159.x. [DOI] [PubMed] [Google Scholar]
- 10.Hung T, Sievenpiper JL, Marchie A, Kendall CW, Jenkins DJ. Fat versus carbohydrate in insulin resistance, obesity, diabetes and cardiovascular disease. Curr Opin Clin Nutr Metab Care. 2003;6:165–76. doi: 10.1097/00075197-200303000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Bjorkegren J, Silveira A, Boquist S, et al. Post-prandial enrichment of remnant lipoproteins with apoC-I in healthy normolipidemic men with early asymptomatic atherosclerosis. Arterioscler Thromb Vasc Biol. 2002;22:1470–4. doi: 10.1161/01.atv.0000029972.42487.42. [DOI] [PubMed] [Google Scholar]
- 12.Boquist S, Karpe F, Danell-Toverud K, Hamsten A. Effects of atorvastatin on post-prandial plasma lipoproteins in postinfarction patients with combined hyperlipidaemia. Atherosclerosis. 2002;162:163–70. doi: 10.1016/s0021-9150(01)00689-x. [DOI] [PubMed] [Google Scholar]
- 13.Syvanne M, Hilden H, Taskinen MR. Abnormal metabolism of post-prandial lipoproteins in patients with non-insulin-dependent diabetes mellitus is not related to coronary artery disease. J Lipid Res. 1994;35:15–26. [PubMed] [Google Scholar]
- 14.Blomhoff R. Transport and metabolism of vitamin A. Nutr Rev. 1994;52:S13–23. doi: 10.1111/j.1753-4887.1994.tb01382.x. [DOI] [PubMed] [Google Scholar]
- 15.Cohn JS, Johnson EJ, Miller JS, et al. Contribution of apo B-48 and apo B-100 triglyceride-rich lipoproteins (TRL) to post-prandial increase in the plasma concentration of TRL triglycerides and retinyl esters. J Lipid Res. 1993;34:2033–40. [PubMed] [Google Scholar]
- 16.Martins IJ, Hopkins L, Joll CA, Redgrave TG. Interactions between model triacylglycerol rich lipoproteins and high density lipoproteins in rat, rabbit and man. Biochim Biophys Acta. 1991;1081:328–38. doi: 10.1016/0005-2760(91)90290-x. [DOI] [PubMed] [Google Scholar]
- 17.Chester A, Somasekaram A, Tzimina M, et al. The apolipoprotein B mRNA editing complex performs a multifunctional cycle and suppresses nonsense-mediated decay. EMBO J. 2003;22:3971–82. doi: 10.1093/emboj/cdg369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Phillips ML, Pullinger C, Kroes I, et al. A single copy of apolipoprotein B-48 is present on the human chylomicron remnant. J Lipid Res. 1997;38:1170–7. [PubMed] [Google Scholar]
- 19.Milne RW, Blanchette L, Theolis R, Jr, Weech PK, Marcel YL. Monoclonal antibodies distinguish between lipid-dependent and reversible conformational states of human apolipoprotein B. Mol Immunol. 1987;24:435–47. doi: 10.1016/0161-5890(87)90017-4. [DOI] [PubMed] [Google Scholar]
- 20.Smith D, Proctor SD, Mamo JC. A highly sensitive assay for quantitation of apolipoprotein B48 using an antibody to human apolipoprotein B and enhanced chemiluminescence. Ann Clin Biochem. 1997;34:185–9. doi: 10.1177/000456329703400210. [DOI] [PubMed] [Google Scholar]
- 21.Hayashi H, Fujimoto K, Cardelli JA, Nutting DF, Bergstedt S, Tso P. Fat feeding increases size, but not number, of chylomicrons produced by small intestine. Am J Physiol. 1990;259:G709–19. doi: 10.1152/ajpgi.1990.259.5.G709. [DOI] [PubMed] [Google Scholar]
- 22.Isherwood SG, Williams CM, Gould BJ. Apolipoprotein B-48 as a marker for chylomicrons and their remnants: studies in the post-prandial state. Proc Nutr Soc. 1997;56:497–505. doi: 10.1079/pns19970050. [DOI] [PubMed] [Google Scholar]
- 23.Karpe F, Hellenius ML, Hamsten A. Differences in post-prandial concentrations of very-low-density lipoprotein and chylomicron remnants between normotriglyceridemic and hypertriglyceridemic men with and without coronary heart disease. Metabolism. 1999;48:301–7. doi: 10.1016/s0026-0495(99)90076-8. [DOI] [PubMed] [Google Scholar]
- 24.Phillips C, Madigan C, Owens D, Collins P, Tomki GH. Defective chylomicron synthesis as a cause of delayed particle clearance in diabetes? International J Exper Diabetes Res. 2002;3:171–8. doi: 10.1080/15604280214277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schreuder PC, Twickler TB, Wang T, Nakajima K, Erkelens DW, Dallinga-Thie GM. Isolation of remnant particles by immunoseparation: a new approach for investigation of post-prandial lipoprotein metabolism in normolipidemic subjects. Atherosclerosis. 2001;157:145–50. doi: 10.1016/s0021-9150(00)00666-3. [DOI] [PubMed] [Google Scholar]
- 26.Funada J, Sekiya M, Hamada M, Hiwada K. Postprandial elevation of remnant lipoprotein leads to endothelial dysfunction. Circ J. 2002;66:127–32. doi: 10.1253/circj.66.127. [DOI] [PubMed] [Google Scholar]
- 27.Kugiyama K, Doi H, Takazoe K, et al. Remnant lipoprotein levels in fasting serum predict coronary events in patients with coronary artery disease. Circulation. 1999;99:2858–60. doi: 10.1161/01.cir.99.22.2858. [DOI] [PubMed] [Google Scholar]
- 28.Qiao Q, Pyorala K, Pyorala M, et al. Two-hour glucose is a better risk predictor for incident coronary heart disease and cardiovascular mortality than fasting glucose. Eur Heart J. 2002;23:1267–75. doi: 10.1053/euhj.2001.3113. [DOI] [PubMed] [Google Scholar]
- 29.Cohen JC, Berger GM. Effects of glucose ingestion on post-prandial lipemia and triglyceride clearance in humans. J Lipid Res. 1990;31:597–602. [PubMed] [Google Scholar]
- 30.Thomsen C, Storm H, Holst JJ, Hermansen K. Differential effects of saturated and monounsaturated fats on post-prandial lipemia and glucagon-like peptide 1 responses in patients with type 2 diabetes. Am J Clin Nutr. 2003;77:605–11. doi: 10.1093/ajcn/77.3.605. [DOI] [PubMed] [Google Scholar]
- 31.Slivkoff-Clarck KM, James AP, Kerr D, Soares MJ, Mamo JCL. The effect of diet standardization on post-prandial chylomicron response. Atherosclerosis Supps. 2003;4:231. [Google Scholar]
- 32.Groot PH, van Stiphout WA, Krauss XH, et al. Post-prandial lipoprotein metabolism in normolipidemic men with and without coronary artery disease. Arterioscler Thromb. 1991;11:653–62. doi: 10.1161/01.atv.11.3.653. [DOI] [PubMed] [Google Scholar]
- 33.Patsch JR, Miesenbock G, Hopferwieser T, et al. Relation of triglyceride metabolism and coronary artery disease. Studies in the post-prandial state. Arterioscler Thromb. 1992;12:1336–45. doi: 10.1161/01.atv.12.11.1336. [DOI] [PubMed] [Google Scholar]
- 34.Brown SA, Chambless LE, Sharrett AR, Gotto AM, Jr, Patsch W. Postprandial lipemia: reliability in an epidemiologic field study. Am J Epidemiol. 1992;136:538–45. doi: 10.1093/oxfordjournals.aje.a116531. [DOI] [PubMed] [Google Scholar]
- 35.Syeda F, Senault C, Delplanque B, et al. Post-prandial variations in the cholesteryl ester transfer protein activity, phospholipid transfer protein activity and plasma cholesterol efflux capacity in normolipidemic men. Nutr Metab Cardiovasc Dis. 2003;13:28–36. doi: 10.1016/s0939-4753(03)80165-5. [DOI] [PubMed] [Google Scholar]
- 36.Chan DC, Watts GF, Barrett PHR, Mamo JC, Redgrave TG. Markers of triglyceride-rich lipoprotein remnant metabolism in visceral obesity. Clin Chem. 2002;48:278–83. [PubMed] [Google Scholar]
- 37.Groener JE, Scheek LM, van Ramshorst E, Krauss XH, van Tol A. Delayed increase in high density lipoprotein-phospholipids after ingestion of a fat load in normolipidemic patients with coronary artery disease. Atherosclerosis. 1998;137:311–9. doi: 10.1016/s0021-9150(97)00287-6. [DOI] [PubMed] [Google Scholar]
- 38.Benlian P, De Gennes JL, Foubert L, Zhang H, Gagne SE, Hayden M. Premature atherosclerosis in patients with familial chylomicronemia caused by mutations in the lipoprotein lipase gene. New Engl J M. 1996;335:848–54. doi: 10.1056/NEJM199609193351203. [DOI] [PubMed] [Google Scholar]
- 39.Lundman P, Eriksson MJ, Stuhlinger M, Cooke JP, Hamsten A, Tornvall P. Mild-to-moderate hypertriglyceridemia in young men is associated with endothelial dysfunction and increased plasma concentrations of asymmetric dimethylarginine. J Am Coll Cardiol. 2001;38:111–6. doi: 10.1016/s0735-1097(01)01318-3. [DOI] [PubMed] [Google Scholar]
- 40.Tanaka A, Ai M, Kobayashi Y, Tamura M, Shimokado K, Numano F. Metabolism of triglyceride-rich lipoproteins and their role in atherosclerosis. Ann NYAcad Sci. 2001;947:207–12. doi: 10.1111/j.1749-6632.2001.tb03942.x. [DOI] [PubMed] [Google Scholar]
- 41.Shaikh M, Martini S, Quiney JR, et al. Modified plasma-derived lipoproteins in human atherosclerotic plaques. Atherosclerosis. 1988;69:165–72. doi: 10.1016/0021-9150(88)90011-1. [DOI] [PubMed] [Google Scholar]
- 42.Brenninkmeijer BJ, Stuyt PM, Demacker PN, Stalenhoef AF, van 't Laar A. Catabolism of chylomicron remnants in normolipidemic subjects in relation to the apoprotein E phenotype. J Lipid Res. 1987;28:361–70. [PubMed] [Google Scholar]
- 43.Fayad ZA, Fallon JT, Shinnar M, et al. Noninvasive in vivo high-resolution magnetic resonance imaging of atherosclerotic lesions in genetically engineered mice. Circulation. 1998;98:1541–7. doi: 10.1161/01.cir.98.15.1541. [DOI] [PubMed] [Google Scholar]
- 44.Proctor SD, Pabla CK, Mamo JC. Arterial intimal retention of pro-atherogenic lipoproteins in insulin deficient rabbits and rats. Atherosclerosis. 2000;149:315–22. doi: 10.1016/s0021-9150(99)00341-x. [DOI] [PubMed] [Google Scholar]
- 45.Cohn JS. Post-prandial lipemia: emerging evidence for atherogenicity of remnant lipoproteins. Can J Cardiol. 1998;14:18B–27B. [PubMed] [Google Scholar]
- 46.Chung BH, Segrest JP. Cytotoxicity of remnants of triglyceride-rich lipoproteins: an atherogenic insult? Adv Exp Med Biol. 1991;285:341–51. doi: 10.1007/978-1-4684-5904-3_42. [DOI] [PubMed] [Google Scholar]
- 47.Le Couteur DG, Fraser R, Cogger VC, McLean AJ. Hepatic pseudocapillarisation and atherosclerosis in ageing. Lancet. 2002;359:1612–5. doi: 10.1016/S0140-6736(02)08524-0. [DOI] [PubMed] [Google Scholar]
- 48.McLean AJ, Cogger VC, Chong GC, et al. Age-related pseudocapillarization of the human liver. J Pathol. 2003;200:112–7. doi: 10.1002/path.1328. [DOI] [PubMed] [Google Scholar]
- 49.Krasinski SD, Cohn JS, Schaefer EJ, Russell RM. Postprandial plasma retinyl ester response is greater in older subjects compared with younger subjects. Evidence for delayed plasma clearance of intestinal lipoproteins. J Clin Invest. 1990;85:883–92. doi: 10.1172/JCI114515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Willnow TE. Mechanisms of hepatic chylomicron remnant clearance. Diabetic Med. 1997;14:S75–S80. doi: 10.1002/(sici)1096-9136(199708)14:3+<s75::aid-dia449>3.3.co;2-0. [DOI] [PubMed] [Google Scholar]
- 51.Mamo JC, Watts GF, Barrett PHR, Smith D, James AP, Pal S. Post-prandial dyslipidemia in men with visceral obesity: an effect of reduced LDL receptor expression? Am J Physiol. 2001;281:E626–E32. doi: 10.1152/ajpendo.2001.281.3.E626. [DOI] [PubMed] [Google Scholar]
- 52.Contacos C, Barter PJ, Vrga L, Sullivan DR. Cholesteryl ester transfer in hypercholesterolaemia: fasting and post-prandial studies with and without pravastatin. Atherosclerosis. 1998;141:87–98. doi: 10.1016/s0021-9150(98)00151-8. [DOI] [PubMed] [Google Scholar]
- 53.Lemieux I, Couillard C, Pascot A, et al. The small, dense LDL phenotype as a correlate of postprandial lipemia in men. Atherosclerosis. 2000;153:423–32. doi: 10.1016/s0021-9150(00)00424-x. [DOI] [PubMed] [Google Scholar]
- 54.Dart AM, Cooper B. Independent effects of Apo E phenotype and plasma triglyceride on lipoprotein particle sizes in the fasting and postprandial states. Arterioscler Thromb Vasc Biol. 1999;19:2465–73. doi: 10.1161/01.atv.19.10.2465. [DOI] [PubMed] [Google Scholar]
- 55.Karpe F, Tornvall P, Olivecrona T, Steiner G, Carlson LA, Hamsten A. Composition of human low density lipoprotein: effects of postprandial triglyceride-rich lipoproteins, lipoprotein lipase, hepatic lipase and cholesteryl ester transfer protein. Atherosclerosis. 1993;98:33–49. doi: 10.1016/0021-9150(93)90221-f. [DOI] [PubMed] [Google Scholar]
- 56.Chung BH, Doran S, Liang P, et al. Alcohol-mediated enhancement of post-prandial lipemia: a contributing factor to an increase in plasma HDL and a decrease in risk of cardiovascular disease. Am J Clin Nutr. 2003;78:391–9. doi: 10.1093/ajcn/78.3.391. [DOI] [PubMed] [Google Scholar]
- 57.Groener JE, Scheek LM, van Ramshorst E, Krauss XH, van Tol A. Delayed increase in high density lipoprotein-phospholipids after ingestion of a fat load in normolipidemic patients with coronary artery disease. Atherosclerosis. 1998;137:311–9. doi: 10.1016/s0021-9150(97)00287-6. [DOI] [PubMed] [Google Scholar]
- 58.Hughes TA, Elam MB, Applegate WB, et al. Postprandial lipoprotein responses in hypertriglyceridemic subjects with and without cardiovascular disease. Metabolism. 1995;44:1082–98. doi: 10.1016/0026-0495(95)90108-6. [DOI] [PubMed] [Google Scholar]
- 59.Durlach V, Attia N, Zahouani A, Leutenegger M, Girard-Globa A. Postprandial cholesteryl ester transfer and high density lipoprotein composition in normotriglyceridemic non-insulin-dependent diabetic patients. Atherosclerosis. 1996;120:155–65. doi: 10.1016/0021-9150(95)05697-1. [DOI] [PubMed] [Google Scholar]
- 60.Sullivan DR, Lam CW, Jessup W, Dean RT, Hensley WJ. Post-prandial changes in apolipoprotein(a) concentration of triglyceride-rich lipoproteins can be reproduced by in vitro incubation: implications for underlying mechanism. Atherosclerosis. 1993;103:139–47. doi: 10.1016/0021-9150(93)90257-u. [DOI] [PubMed] [Google Scholar]
- 61.Sullivan D, Lam C, Vrga L, Contacos C, Jessup W, Hensley W. Preferential redistribution of apolipoprotein (a) isoforms between post-prandial lipoprotein fractions in plasma. Atherosclerosis. 1994;109:280. [Google Scholar]
- 62.Cohn JS, Lam CW, Sullivan DR, Hensley WJ. Plasma lipoprotein distribution of apolipoprotein(a) in the fed and fasted states. Atherosclerosis. 1991;90:59–66. doi: 10.1016/0021-9150(91)90244-w. [DOI] [PubMed] [Google Scholar]
- 63.Lundstam U, Herlitz J, Karlsson T, Linden T, Wiklund O. Serum lipids, lipoprotein(a) level, and apolipoprotein(a) isoforms as prognostic markers in patients with coronary heart disease. J Intern Med. 2002;251:111–8. doi: 10.1046/j.1365-2796.2002.00937.x. [DOI] [PubMed] [Google Scholar]
- 64.Bersot TP, Innerarity TL, Pitas RE, Rall SC, Jr, Weisgraber KH, Mahley RW. Fat feeding in humans induces lipoproteins of density less than 1.006 that are enriched in apolipoprotein [a] and that cause lipid accumulation in macrophages. J Clin Invest. 1986;77:622–30. doi: 10.1172/JCI112345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mikhailenko I, Battey FD, Migliorini M, et al. Recognition of alpha 2-macroglobulin by the low density lipoprotein receptor-related protein requires the cooperation of two ligand binding cluster regions. J Biol Chem. 2001;276:39484–91. doi: 10.1074/jbc.M104382200. [DOI] [PubMed] [Google Scholar]
- 66.Knight BL. Lp(a) catabolism in hypercholesterolaemic individuals. Chem Physics Lipids. 67–68:233–9. doi: 10.1016/0009-3084(94)90142-2. [DOI] [PubMed] [Google Scholar]
- 67.Beisiegal U, Weber W, Gudrun I, Herz J, Stanley K. The LDL-receptor-related protein, LRP, is an apolipoprotein E-binding protein. Nature. 1989;341:162–3. doi: 10.1038/341162a0. [DOI] [PubMed] [Google Scholar]
- 68.Vogel RA. Brachial artery ultrasound: a noninvasive tool in the assessment of triglyceride-rich lipoproteins. Clin Cardiol. 1999;22:II34–9. doi: 10.1002/clc.4960221407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Williams MJ, Sutherland WH, McCormick MP, Yeoman D, de Jong SA, Walker RJ. Normal endothelial function after meals rich in olive or safflower oil previously used for deep frying. Nutr Metab Cardiovasc Dis. 2001;11:147–52. [PubMed] [Google Scholar]
- 70.Jagla A, Schrezenmeir J. Postprandial triglycerides and endothelial function. Exper Clin Endocrinol Diabetes. 2001;109:S533–S47. doi: 10.1055/s-2001-15116. [DOI] [PubMed] [Google Scholar]
- 71.Lupattelli G, Pasqualini L, Siepi D, et al. Increased postprandial lipemia in patients with normolipemic peripheral arterial disease. Am Heart J. 2002;143:733–8. doi: 10.1067/mhj.2002.120302. [DOI] [PubMed] [Google Scholar]
- 72.Nestel PJ, Shige H, Pomeroy S, Cehun M, Chin-Dusting J. Post-prandial remnant lipids impair arterial compliance. J Am Coll Cardiol. 2001;37:1929–35. doi: 10.1016/s0735-1097(01)01251-7. [DOI] [PubMed] [Google Scholar]
- 73.Lee IK, Kim HS, Bae JH. Endothelial dysfunction: its relationship with acute hyperglycaemia and hyperlipidemia. Int J Clin Pract Suppl. 2002;129:59–64. [PubMed] [Google Scholar]
- 74.Ling L, Zhao SP, Gao M, Zhou QC, Li YL, Xia B. Vitamin C preserves endothelial function in patients with coronary heart disease after a high-fat meal. Clin Cardiol. 2002;25:219–24. doi: 10.1002/clc.4950250505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhao SP, Liu L, Gao M, Zhou QC, Li YL, Xia B. Impairment of endothelial function after a high-fat meal in patients with coronary artery disease. Coron Artery Dis. 2001;12:561–5. doi: 10.1097/00019501-200111000-00006. [DOI] [PubMed] [Google Scholar]
- 76.Siepi D, Marchesi S, Lupattelli G, et al. Postprandial endothelial impairment and reduced glutathione levels in postmenopausal women. Ann Nutr Metab. 2002;46:32–7. doi: 10.1159/000046750. [DOI] [PubMed] [Google Scholar]
- 77.de Roos NM, Siebelink E, Bots ML, van Tol A, Schouten EG, Katan MB. Trans monounsaturated fatty acids and saturated fatty acids have similar effects on post-prandial flow-mediated vasodilation. Eur J Clin Nutr. 2002;56:674–9. doi: 10.1038/sj.ejcn.1601377. [DOI] [PubMed] [Google Scholar]
- 78.van Dijk RA, Bakker SJ, Scheffer PG, Heine RJ, Stehouwer CD. Associations of metabolic variables with arterial stiffness in type 2 diabetes mellitus: focus on insulin sensitivity and postprandial triglyceridaemia. Eur J Clin Invest. 2003;33:307–15. doi: 10.1046/j.1365-2362.2003.01137.x. [DOI] [PubMed] [Google Scholar]
- 79.Moore JE, Jr, Ku DN. Pulsatile velocity measurements in a model of the human abdominal aorta under simulated exercise and postprandial conditions. J Biomechan Engin. 1994;116:107–11. doi: 10.1115/1.2895692. [DOI] [PubMed] [Google Scholar]
- 80.Schinkovitz A, Dittrich P, Wascher TC. Effects of a high-fat meal on resistance vessel reactivity and on indicators of oxidative stress in healthy volunteers. Clin Physiol. 2001;21:404–10. doi: 10.1046/j.1365-2281.2001.00341.x. [DOI] [PubMed] [Google Scholar]
- 81.Raitakari OT, Lai N, Griffiths K, McCredie R, Sullivan D, Celermajer DS. Enhanced peripheral vasodilation in humans after a fatty meal. J Am Coll Cardiol. 2000;36:417–22. doi: 10.1016/s0735-1097(00)00758-0. [DOI] [PubMed] [Google Scholar]
- 82.Teno S, Uto Y, Nagashima H, et al. Association of postprandial hypertriglyceridemia and carotid intima-media thickness in patients with type 2 diabetes. Diabetes Care. 2000;23:1401–6. doi: 10.2337/diacare.23.9.1401. [DOI] [PubMed] [Google Scholar]
- 83.Summers LK, Samra JS, Frayn KN. Impaired postprandial tissue regulation of blood flow in insulin resistance: a determinant of cardiovascular risk? Atherosclerosis. 1999;147:11–5. doi: 10.1016/s0021-9150(99)00172-0. [DOI] [PubMed] [Google Scholar]
- 84.Cooper MB, Tan KC, Hales CN, Betteridge DJ. Postprandial lipid metabolism and beta-cell function in non-insulin-dependent (type 2) diabetes mellitus after a mixed meal with a high fat content. Diabetic Med. 1996;13:816–27. doi: 10.1002/(SICI)1096-9136(199609)13:9<816::AID-DIA183>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 85.Schrezenmeir J, Keppler I, Fenselau S, et al. The phenomenon of a high triglyceride response to an oral lipid load in healthy subjects and its link to the metabolic syndrome. Ann NY AcadSci. 1993;683:302–14. doi: 10.1111/j.1749-6632.1993.tb35721.x. [DOI] [PubMed] [Google Scholar]
- 86.Bonora E, Muggeo M. Postprandial blood glucose as a risk factor for cardiovascular disease in Type II diabetes: the epidemiological evidence. Diabetologia. 2001;44:2107–14. doi: 10.1007/s001250100020. [DOI] [PubMed] [Google Scholar]
- 87.Haynes WG. Hyperhomocysteinemia, vascular function and atherosclerosis: effects of vitamins. Cardiovasc Drugs Ther. 2002;16:391–9. doi: 10.1023/a:1022130217463. [DOI] [PubMed] [Google Scholar]
- 88.Wilmink HW, Stroes ES, Erkelens WD, et al. Influence of folic acid on postprandial endothelial dysfunction. Arterioscler Thromb Vasc Biol. 2000;20:185–8. doi: 10.1161/01.atv.20.1.185. [DOI] [PubMed] [Google Scholar]
- 89.Adams MR, McCredie R, Jessup W, Robinson J, Sullivan D, Celermajer DS. Oral L-arginine improves endothelium-dependent dilatation and reduces monocyte adhesion to endothelial cells in young men with coronary artery disease. Atherosclerosis. 1997;129:261–9. doi: 10.1016/s0021-9150(96)06044-3. [DOI] [PubMed] [Google Scholar]
- 90.Marchesi S, Lupattelli G, Siepi D, et al. Oral L-arginine administration attenuates postprandial endothelial dysfunction in young healthy males. J Clin Pharm Ther. 2001;26:343–9. doi: 10.1046/j.1365-2710.2001.00362.x. [DOI] [PubMed] [Google Scholar]
- 91.Jeremy RW, McCarron H, Sullivan D. Effects of dietary L-arginine on atherosclerosis and endothelium-dependent vasodilatation in the hypercholesterolemic rabbit. Response according to treatment duration, anatomic site, and sex. Circulation. 1996;94:498–506. doi: 10.1161/01.cir.94.3.498. [DOI] [PubMed] [Google Scholar]
- 92.Nordoy A, Bonaa KH, Sandset PM, Hansen JB, Nilsen H. Relationship between apolipoprotein E polymorphism, post-prandial hyperlipemia and hemostatic variables in patients with combined hyperlipemia. Nutr Metab Cardiovasc Dis. 2000;10:15–23. [PubMed] [Google Scholar]
- 93.Ghaddar HM, Folsom AR, Aleksic N, et al. Correlation of factor VIIa values with factor VII gene polymorphism, fasting and postprandial triglyceride levels, and subclinical carotid atherosclerosis. Circulation. 1998;98:2815–21. doi: 10.1161/01.cir.98.25.2815. [DOI] [PubMed] [Google Scholar]
- 94.Jastrzebska M, Przybycien K, Chelstowski K, Torbus-Lisiecka B, Kornacewicz-Jach Z, Naruszewicz M . Increased levels of factor VII, fibrinogen and activity of plasminogen activator inhibitor during postprandial triglyceridemia in patients with ischemic heart disease confirmed by angiography. Nutr Metab Cardiovasc Dis. 1999;9:33–40. [PubMed] [Google Scholar]
- 95.Roche HM, Black IL, Noone E, Tully AM, Whitehead AS, Gibney MJ. Postprandial factor VII metabolism: the effect of the R353Q and 10 bp polymorphisms. Brit J Nutr. 2000;83:467–72. [PubMed] [Google Scholar]
- 96.Anderson RA, Jones CJ, Goodfellow J. Is the fatty meal a trigger for acute coronary syndromes. Atherosclerosis. 2001;159:9–15. doi: 10.1016/s0021-9150(01)00669-4. [DOI] [PubMed] [Google Scholar]
- 97.Braun D, Gramlich A, Brehme U, Kahle PF, Schmahl FW. Post-prandial lipaemia after a moderate fat challenge in normolipidaemic men with and without coronary artery disease. J Cardiovasc Risk. 1997;4:143–9. [PubMed] [Google Scholar]
- 98.Lupattelli G, Pasqualini L, Siepi D, et al. Increased postprandial lipemia in patients with normolipemic peripheral arterial disease. Am Heart J. 2002;143:733–8. doi: 10.1067/mhj.2002.120302. [DOI] [PubMed] [Google Scholar]
- 99.Meyer E, Westerveld HT, de Ruyter-Meijstek FC, et al. Abnormal postprandial apolipoprotein B-48 and triglyceride responses in normolipidemic women with greater than 70% stenotic coronary artery disease: a case-control study. Atherosclerosis. 1996;124:221–35. doi: 10.1016/0021-9150(96)05832-7. [DOI] [PubMed] [Google Scholar]
- 100.Weintraub MS, Grosskopf I, Rassin T, et al. Clearance of chylomicron remnants in normolipidaemic patients with coronary artery disease: case control study over three years. Brit Med J. 1996;312:936–9. doi: 10.1136/bmj.312.7036.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kofoed SC, Gronholdt ML, Bismuth J, Wilhjelm JE, Sillesen H, Nordestgaard BG. Echolucent, rupture-prone carotid plaques associated with elevated triglyceride-rich lipoproteins, particularly in women. J Vasc Surg. 2002;36:783–92. [PubMed] [Google Scholar]
- 102.Bae JH, Bassenge E, Lee HJ, et al. Impact of postprandial hypertriglyceridemia on vascular responses in patients with coronary artery disease: effects of ACE inhibitors and fibrates. Atherosclerosis. 2001;158:165–71. doi: 10.1016/s0021-9150(01)00408-7. [DOI] [PubMed] [Google Scholar]
- 103.Westphal S, Gekeler GH, Dierkes J, Wieland H, Luley C. A free fatty acid tolerance test identifies patients with coronary artery disease among individuals with a low conventional coronary risk profile. Heart Vessels. 2002;16:79–85. doi: 10.1007/s003800200000. [DOI] [PubMed] [Google Scholar]
- 104.Sharrett AR, Chambless LE, Heiss G, Paton CC, Patsch W. Association of postprandial triglyceride and retinyl palmitate responses with asymptomatic carotid artery atherosclerosis in middle-aged men and women. The Atherosclerosis Risk in Communities (ARIC) Study. Arterioscler Thromb Vasc Biol. 1995;15:2122–9. doi: 10.1161/01.atv.15.12.2122. [DOI] [PubMed] [Google Scholar]
- 105.Karpe F, Steiner G, Uffelman K, Olivecrona T, Hamsten A. Postprandial lipoproteins and progression of coronary atherosclerosis. Atherosclerosis. 1994;106:83–97. doi: 10.1016/0021-9150(94)90085-x. [DOI] [PubMed] [Google Scholar]
- 106.Uiterwaal CS, Grobbee DE, Witteman JC, et al. Postprandial triglyceride response in young adult men and familial risk for coronary atherosclerosis. Ann Intern Med. 1994;121:576–83. doi: 10.7326/0003-4819-121-8-199410150-00004. [DOI] [PubMed] [Google Scholar]
- 107.Karpe F, Boquist S, Tang R, Bond GM, de Faire U, Hamsten A. Remnant lipoproteins are related to intima-media thickness of the carotid artery independently of LDL cholesterol and plasma triglycerides. J Lipid Res. 2001;42:17–21. [PubMed] [Google Scholar]
- 108.Schaefer EJ, Audelin MC, McNamara JR, et al. Comparison of fasting and postprandial plasma lipoproteins in subjects with and without coronary heart disease. Am J Cardiol. 2001;88:1129–33. doi: 10.1016/s0002-9149(01)02047-1. [DOI] [PubMed] [Google Scholar]
- 109.Smith D, Watts GF, Dane-Stewart C, Mamo JC. Post-prandial chylomicron response may be predicted by a single measurement of plasma apolipoprotein B48 in the fasting state. Eur J Clin Invest. 1999;29:204–9. doi: 10.1046/j.1365-2362.1999.00431.x. [DOI] [PubMed] [Google Scholar]