Figure 1.
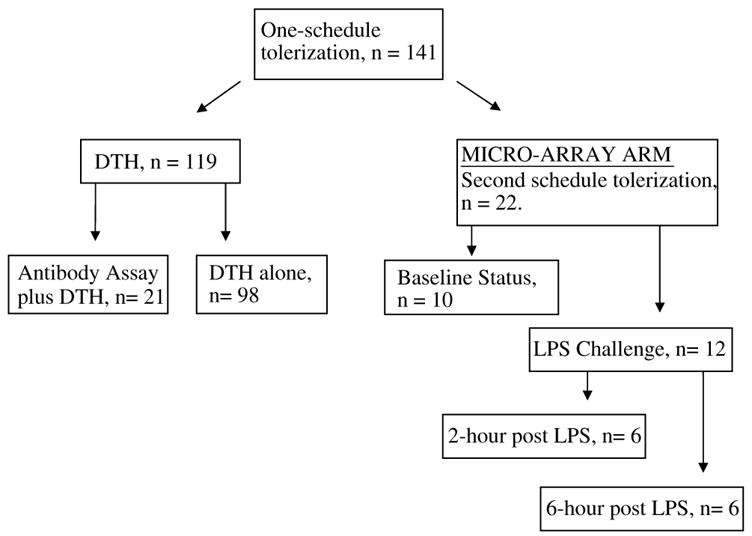
Allocation of animals according to study group. Study animals (n = 141) received one schedule of intranasal tolerization with E-selectin or PBS. Delayed-type hypersensitivity studies were performed assess antibody generation (n = 21) or efficacy of E-selectin doses (n = 98). The other animals (n = 22) were treated on a second tolerization schedule and used for the microarray studies.
