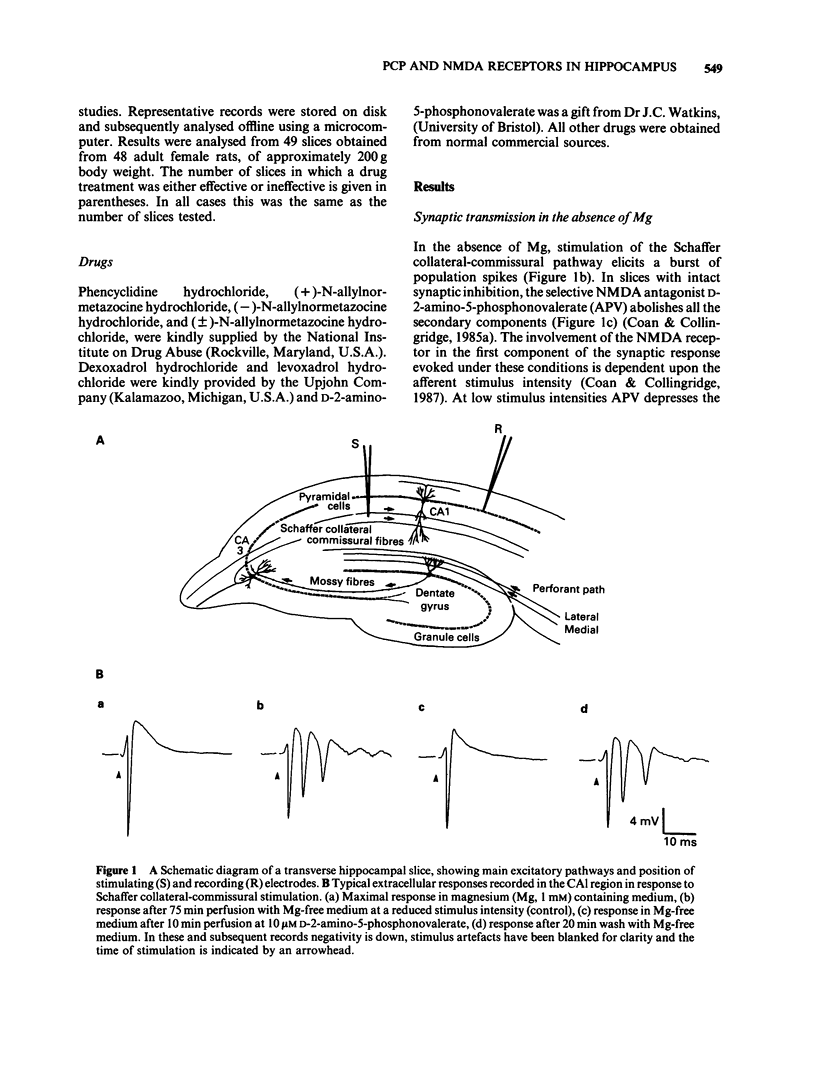
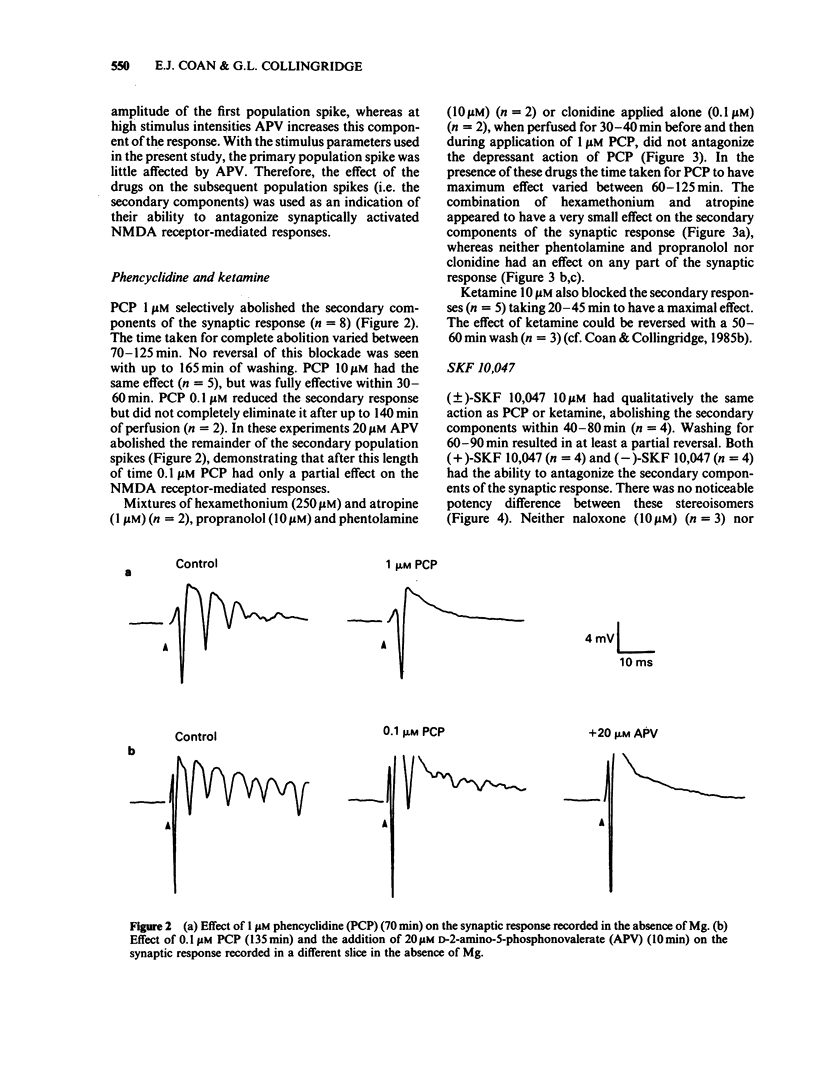
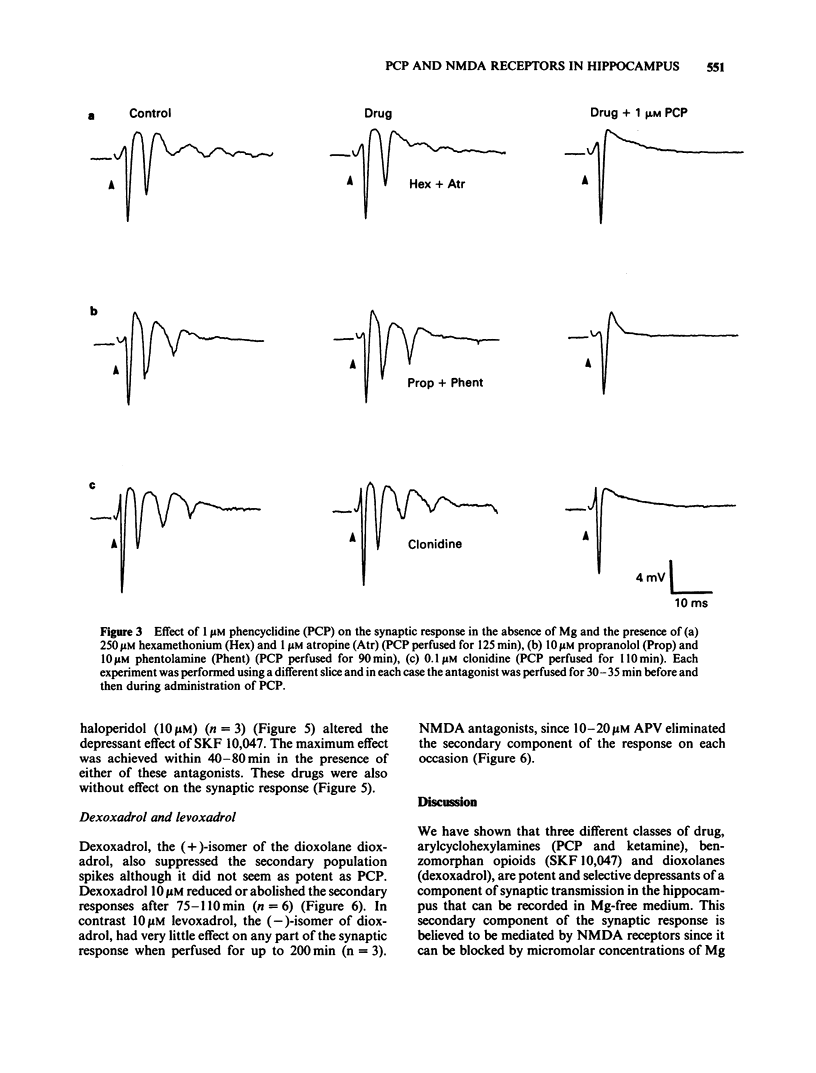
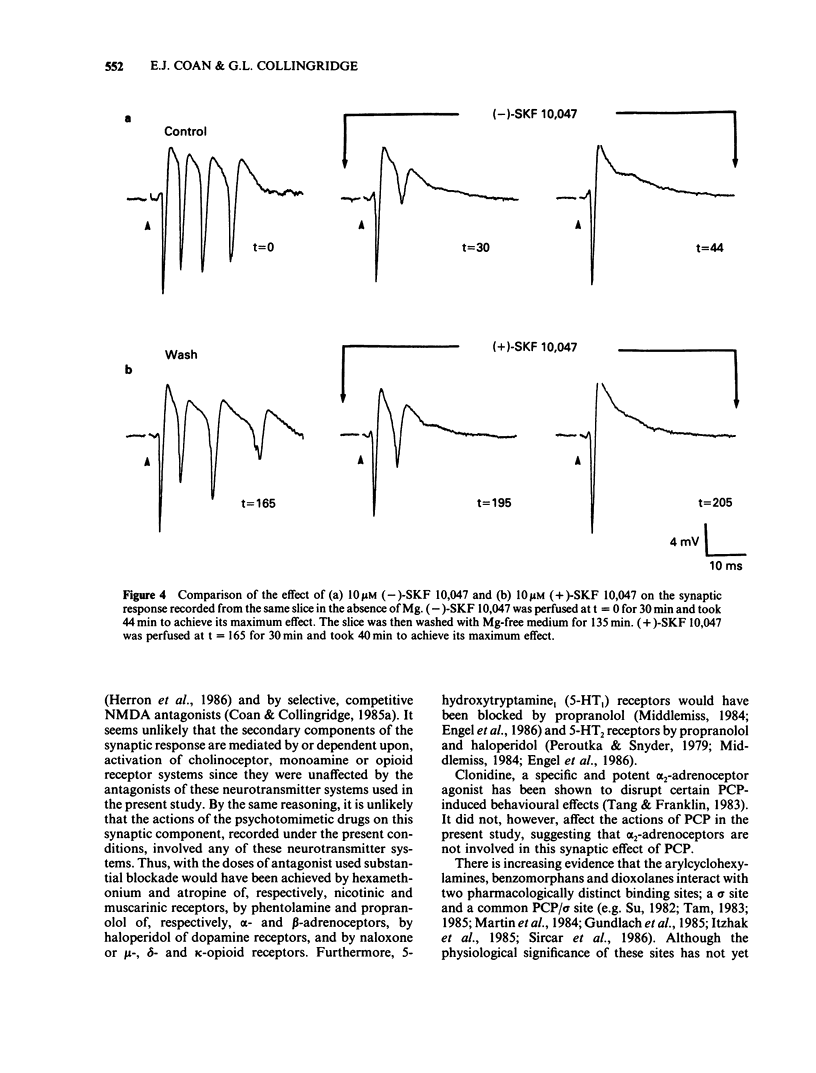
Abstract
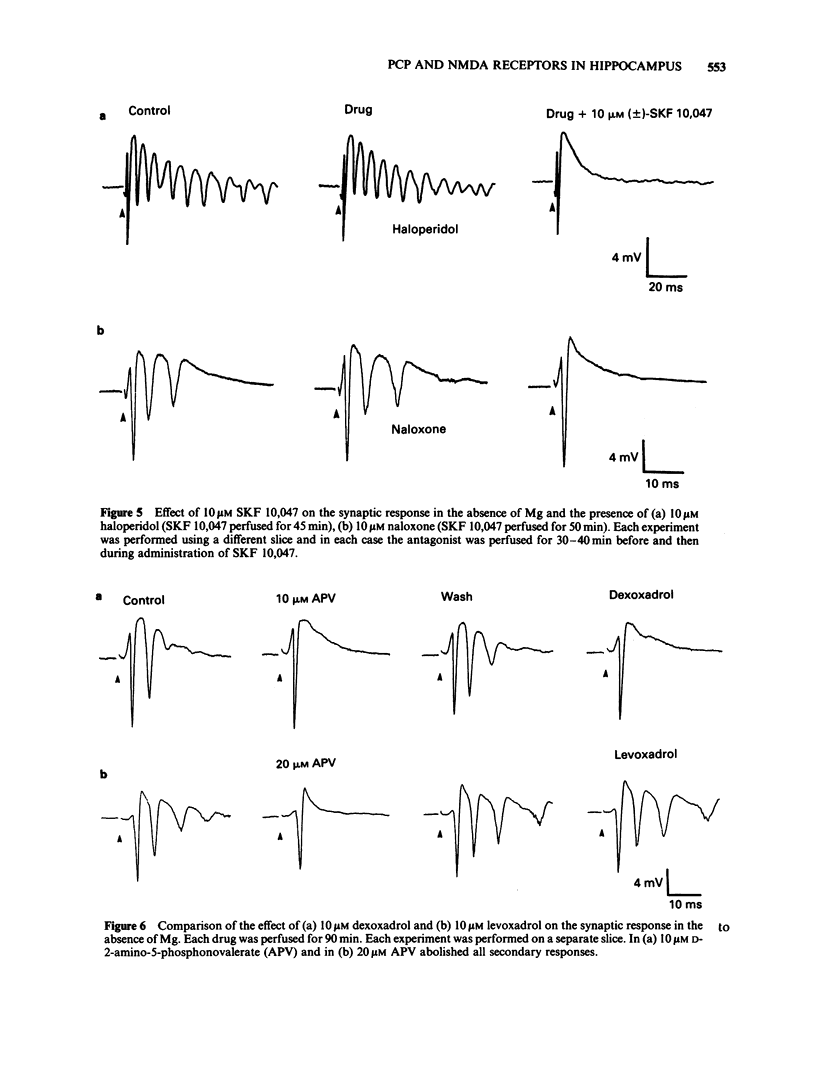
The effects of representative drugs from three classes of psychotomimetic compounds (arylcyclohexylamines, benzomorphan opioids and dioxolanes) have been examined on synaptic transmission at an identified monosynaptic pathway in rat hippocampal slices. The compounds tested were phencyclidine (PCP) and ketamine, the racemate and isomers of SKF 10,047 (N-allylnormetazocine), and the isomers of dioxadrol (dexoxadrol and levoxadrol). In the absence of added magnesium ions (Mg) in the perfusion medium low frequency stimulation of the Schaffer collateral-commissural pathway evoked a burst of population spikes in the CA1 cell body region. The secondary components of this response could be abolished by the selective N-methyl-D-aspartate (NMDA) antagonist D-2-amino-5-phosphonovalerate (APV). PCP (1 microM) or ketamine (10 microM) selectively blocked the secondary components of the synaptic response. The effect of PCP was neither mimicked nor prevented by hexamethonium and atropine, phentolamine and propranolol, or clonidine and was therefore unlikely to involve cholinergic or adrenergic neurotransmitter systems. The sigma opiate, (+/-)-SKF 10,047 (10 microM) also abolished selectively the secondary components of the synaptic response. There was no apparent difference between the potency of the stereoisomers of this compound. The action of (+/-)-SKF 10,047 was not affected by either naloxone or haloperidol, indicating that this effect did not involve opioid receptors or the haloperidol-sensitive sigma site. Dexoxadrol (10 microM), but not levoxadrol (10 microM), also selectively blocked the secondary components of the synaptic response. It is concluded that these psychotomimetic agents can block an NMDA receptor-mediated component of synaptic transmission in the hippocampus and that this effect is mediated by a specific PCP/sigma site.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aanonsen L. M., Wilcox G. L. Phencyclidine selectively blocks a spinal action of N-methyl-D-aspartate in mice. Neurosci Lett. 1986 Jun 18;67(2):191–197. doi: 10.1016/0304-3940(86)90396-4. [DOI] [PubMed] [Google Scholar]
- Anis N. A., Berry S. C., Burton N. R., Lodge D. The dissociative anaesthetics, ketamine and phencyclidine, selectively reduce excitation of central mammalian neurones by N-methyl-aspartate. Br J Pharmacol. 1983 Jun;79(2):565–575. doi: 10.1111/j.1476-5381.1983.tb11031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ault B., Evans R. H., Francis A. A., Oakes D. J., Watkins J. C. Selective depression of excitatory amino acid induced depolarizations by magnesium ions in isolated spinal cord preparations. J Physiol. 1980 Oct;307:413–428. doi: 10.1113/jphysiol.1980.sp013443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry S. C., Anis N. A., Lodge D. The effect of the dioxolanes on amino acid induced excitation in the mammalian spinal cord. Brain Res. 1984 Jul 30;307(1-2):85–90. doi: 10.1016/0006-8993(84)90463-3. [DOI] [PubMed] [Google Scholar]
- Berry S. C., Dawkins S. L., Lodge D. Comparison of sigma- and kappa-opiate receptor ligands as excitatory amino acid antagonists. Br J Pharmacol. 1984 Sep;83(1):179–185. doi: 10.1111/j.1476-5381.1984.tb10133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN G., ENSOR C. R., RUSSELL D., BOHNER B. The pharmacology of 1-(1-phenylcyclohexyl) piperidine-HCl. J Pharmacol Exp Ther. 1959 Nov;127:241–250. [PubMed] [Google Scholar]
- Cahusac P. M., Evans R. H., Hill R. G., Rodriquez R. E., Smith D. A. The behavioural effects of an N-methylaspartate receptor antagonist following application to the lumbar spinal cord of conscious rats. Neuropharmacology. 1984 Jul;23(7A):719–724. doi: 10.1016/0028-3908(84)90102-3. [DOI] [PubMed] [Google Scholar]
- Coan E. J., Collingridge G. L. Magnesium ions block an N-methyl-D-aspartate receptor-mediated component of synaptic transmission in rat hippocampus. Neurosci Lett. 1985 Jan 7;53(1):21–26. doi: 10.1016/0304-3940(85)90091-6. [DOI] [PubMed] [Google Scholar]
- Collingridge G. L., Kehl S. J., McLennan H. Excitatory amino acids in synaptic transmission in the Schaffer collateral-commissural pathway of the rat hippocampus. J Physiol. 1983 Jan;334:33–46. doi: 10.1113/jphysiol.1983.sp014478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras P. C., Quirion R., O'Donohue T. L. Autoradiographic distribution of phencyclidine receptors in the rat brain using [3H]1-(1-(2-thienyl)cyclohexyl)piperidine ([3H]TCP). Neurosci Lett. 1986 Jun 18;67(2):101–106. doi: 10.1016/0304-3940(86)90380-0. [DOI] [PubMed] [Google Scholar]
- Croucher M. J., Collins J. F., Meldrum B. S. Anticonvulsant action of excitatory amino acid antagonists. Science. 1982 May 21;216(4548):899–901. doi: 10.1126/science.7079744. [DOI] [PubMed] [Google Scholar]
- Crunelli V., Mayer M. L. Mg2+ dependence of membrane resistance increases evoked by NMDA in hippocampal neurones. Brain Res. 1984 Oct 8;311(2):392–396. doi: 10.1016/0006-8993(84)90107-0. [DOI] [PubMed] [Google Scholar]
- Dingledine R., Hynes M. A., King G. L. Involvement of N-methyl-D-aspartate receptors in epileptiform bursting in the rat hippocampal slice. J Physiol. 1986 Nov;380:175–189. doi: 10.1113/jphysiol.1986.sp016279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingledine R. N-methyl aspartate activates voltage-dependent calcium conductance in rat hippocampal pyramidal cells. J Physiol. 1983 Oct;343:385–405. doi: 10.1113/jphysiol.1983.sp014899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchen M. R., Burton N. R., Biscoe T. J. An intracellular study of the interactions of N-methyl-DL-aspartate with ketamine in the mouse hippocampal slice. Brain Res. 1985 Sep 2;342(1):149–153. doi: 10.1016/0006-8993(85)91364-2. [DOI] [PubMed] [Google Scholar]
- Engel G., Göthert M., Hoyer D., Schlicker E., Hillenbrand K. Identity of inhibitory presynaptic 5-hydroxytryptamine (5-HT) autoreceptors in the rat brain cortex with 5-HT1B binding sites. Naunyn Schmiedebergs Arch Pharmacol. 1986 Jan;332(1):1–7. doi: 10.1007/BF00633189. [DOI] [PubMed] [Google Scholar]
- Gundlach A. L., Largent B. L., Snyder S. H. Phencyclidine and sigma opiate receptors in brain: biochemical and autoradiographical differentiation. Eur J Pharmacol. 1985 Jul 31;113(3):465–466. doi: 10.1016/0014-2999(85)90100-1. [DOI] [PubMed] [Google Scholar]
- Hampton R. Y., Medzihradsky F., Woods J. H., Dahlstrom P. J. Stereospecific binding of 3H-phencyclidine in brain membranes. Life Sci. 1982 Jun 21;30(25):2147–2154. doi: 10.1016/0024-3205(82)90288-0. [DOI] [PubMed] [Google Scholar]
- Harrison N. L., Simmonds M. A. Quantitative studies on some antagonists of N-methyl D-aspartate in slices of rat cerebral cortex. Br J Pharmacol. 1985 Feb;84(2):381–391. doi: 10.1111/j.1476-5381.1985.tb12922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herron C. E., Lester R. A., Coan E. J., Collingridge G. L. Frequency-dependent involvement of NMDA receptors in the hippocampus: a novel synaptic mechanism. Nature. 1986 Jul 17;322(6076):265–268. doi: 10.1038/322265a0. [DOI] [PubMed] [Google Scholar]
- Herron C. E., Williamson R., Collingridge G. L. A selective N-methyl-D-aspartate antagonist depresses epileptiform activity in rat hippocampal slices. Neurosci Lett. 1985 Nov 11;61(3):255–260. doi: 10.1016/0304-3940(85)90473-2. [DOI] [PubMed] [Google Scholar]
- Holtzman S. G. Phencyclidine-like discriminative effects of opioids in the rat. J Pharmacol Exp Ther. 1980 Sep;214(3):614–619. [PubMed] [Google Scholar]
- Honey C. R., Miljkovic Z., MacDonald J. F. Ketamine and phencyclidine cause a voltage-dependent block of responses to L-aspartic acid. Neurosci Lett. 1985 Oct 24;61(1-2):135–139. doi: 10.1016/0304-3940(85)90414-8. [DOI] [PubMed] [Google Scholar]
- Itzhak Y., Hiller J. M., Simon E. J. Characterization of specific binding sites for [3H](d)-N-allylnormetazocine in rat brain membranes. Mol Pharmacol. 1985 Jan;27(1):46–52. [PubMed] [Google Scholar]
- Koek W., Kleer E., Mudar P. J., Woods J. H. Phencyclidine-like catalepsy induced by the excitatory amino acid antagonist DL-2-amino-5-phosphonovalerate. Behav Brain Res. 1986 Mar;19(3):257–259. doi: 10.1016/0166-4328(86)90026-4. [DOI] [PubMed] [Google Scholar]
- Lacey M. G., Henderson G. Actions of phencyclidine on rat locus coeruleus neurones in vitro. Neuroscience. 1986 Feb;17(2):485–494. doi: 10.1016/0306-4522(86)90261-7. [DOI] [PubMed] [Google Scholar]
- Largent B. L., Gundlach A. L., Snyder S. H. Psychotomimetic opiate receptors labeled and visualized with (+)-[3H]3-(3-hydroxyphenyl)-N-(1-propyl)piperidine. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4983–4987. doi: 10.1073/pnas.81.15.4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge D., Johnston G. A. Effect of ketamine on amino acid-evoked release of acetylcholine from rat cerebral cortex in vitro. Neurosci Lett. 1985 May 23;56(3):371–375. doi: 10.1016/0304-3940(85)90271-x. [DOI] [PubMed] [Google Scholar]
- Loo P., Braunwalder A., Lehmann J., Williams M. Radioligand binding to central phencyclidine recognition sites is dependent on excitatory amino acid receptor agonists. Eur J Pharmacol. 1986 Apr 29;123(3):467–468. doi: 10.1016/0014-2999(86)90726-0. [DOI] [PubMed] [Google Scholar]
- Maragos W. F., Chu D. C., Greenamyre J. T., Penney J. B., Young A. B. High correlation between the localization of [3H]TCP binding and NMDA receptors. Eur J Pharmacol. 1986 Apr 9;123(1):173–174. doi: 10.1016/0014-2999(86)90703-x. [DOI] [PubMed] [Google Scholar]
- Martin B. R., Katzen J. S., Woods J. A., Tripathi H. L., Harris L. S., May E. L. Stereoisomers of [3H]-N-allylnormetazocine bind to different sites in mouse brain. J Pharmacol Exp Ther. 1984 Dec;231(3):539–544. [PubMed] [Google Scholar]
- Martin D., Lodge D. Ketamine acts as a non-competitive N-methyl-D-aspartate antagonist on frog spinal cord in vitro. Neuropharmacology. 1985 Oct;24(10):999–1003. doi: 10.1016/0028-3908(85)90128-5. [DOI] [PubMed] [Google Scholar]
- Middlemiss D. N. Stereoselective blockade at [3H]5-HT binding sites and at the 5-HT autoreceptor by propranolol. Eur J Pharmacol. 1984 Jun 1;101(3-4):289–293. doi: 10.1016/0014-2999(84)90173-0. [DOI] [PubMed] [Google Scholar]
- Nowak L., Bregestovski P., Ascher P., Herbet A., Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984 Feb 2;307(5950):462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Peroutka S. J., Snyder S. H. Multiple serotonin receptors: differential binding of [3H]5-hydroxytryptamine, [3H]lysergic acid diethylamide and [3H]spiroperidol. Mol Pharmacol. 1979 Nov;16(3):687–699. [PubMed] [Google Scholar]
- Shannon H. E. Pharmacological evaluation of N-allynormetazocine (SKF 10,047) on the basis of its discriminative stimulus properties in the rat. J Pharmacol Exp Ther. 1983 Apr;225(1):144–152. [PubMed] [Google Scholar]
- Sircar R., Nichtenhauser R., Ieni J. R., Zukin S. R. Characterization and autoradiographic visualization of (+)-[3H]SKF10,047 binding in rat and mouse brain: further evidence for phencyclidine/"sigma opiate" receptor commonality. J Pharmacol Exp Ther. 1986 May;237(2):681–688. [PubMed] [Google Scholar]
- Snell L. D., Johnson K. M. Antagonism of N-methyl-D-aspartate-induced transmitter release in the rat striatum by phencyclidine-like drugs and its relationship to turning behavior. J Pharmacol Exp Ther. 1985 Oct;235(1):50–57. [PubMed] [Google Scholar]
- Stringer J. L., Greenfield L. J., Hackett J. T., Guyenet P. G. Blockade of long-term potentiation by phencyclidine and sigma opiates in the hippocampus in vivo and in vitro. Brain Res. 1983 Nov 28;280(1):127–138. doi: 10.1016/0006-8993(83)91180-0. [DOI] [PubMed] [Google Scholar]
- Su T. P. Evidence for sigma opioid receptor: binding of [3H]SKF-10047 to etorphine-inaccessible sites in guinea-pig brain. J Pharmacol Exp Ther. 1982 Nov;223(2):284–290. [PubMed] [Google Scholar]
- Tam S. W. (+)-[3H]SKF 10,047, (+)-[3H]ethylketocyclazocine, mu, kappa, delta and phencyclidine binding sites in guinea pig brain membranes. Eur J Pharmacol. 1985 Feb 12;109(1):33–41. doi: 10.1016/0014-2999(85)90536-9. [DOI] [PubMed] [Google Scholar]
- Tam S. W. Naloxone-inaccessible sigma receptor in rat central nervous system. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6703–6707. doi: 10.1073/pnas.80.21.6703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang A. H., Franklin S. R. Disruption of brightness discrimination in a shock avoidance task by phencyclidine and its antagonism in rats. J Pharmacol Exp Ther. 1983 Jun;225(3):503–508. [PubMed] [Google Scholar]
- Vignon J., Privat A., Chaudieu I., Thierry A., Kamenka J. M., Chicheportiche R. [3H]thienyl-phencyclidine ([3H]TCP) binds to two different sites in rat brain. Localization by autoradiographic and biochemical techniques. Brain Res. 1986 Jul 16;378(1):133–141. doi: 10.1016/0006-8993(86)90294-5. [DOI] [PubMed] [Google Scholar]
- Vincent J. P., Kartalovski B., Geneste P., Kamenka J. M., Lazdunski M. Interaction of phencyclidine ("angel dust") with a specific receptor in rat brain membranes. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4678–4682. doi: 10.1073/pnas.76.9.4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zukin R. S., Zukin S. R. Demonstration of [3H]cyclazocine binding to multiple opiate receptor sites. Mol Pharmacol. 1981 Sep;20(2):246–254. [PubMed] [Google Scholar]
- Zukin S. R., Zukin R. S. Specific [3H]phencyclidine binding in rat central nervous system. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5372–5376. doi: 10.1073/pnas.76.10.5372. [DOI] [PMC free article] [PubMed] [Google Scholar]


