Abstract
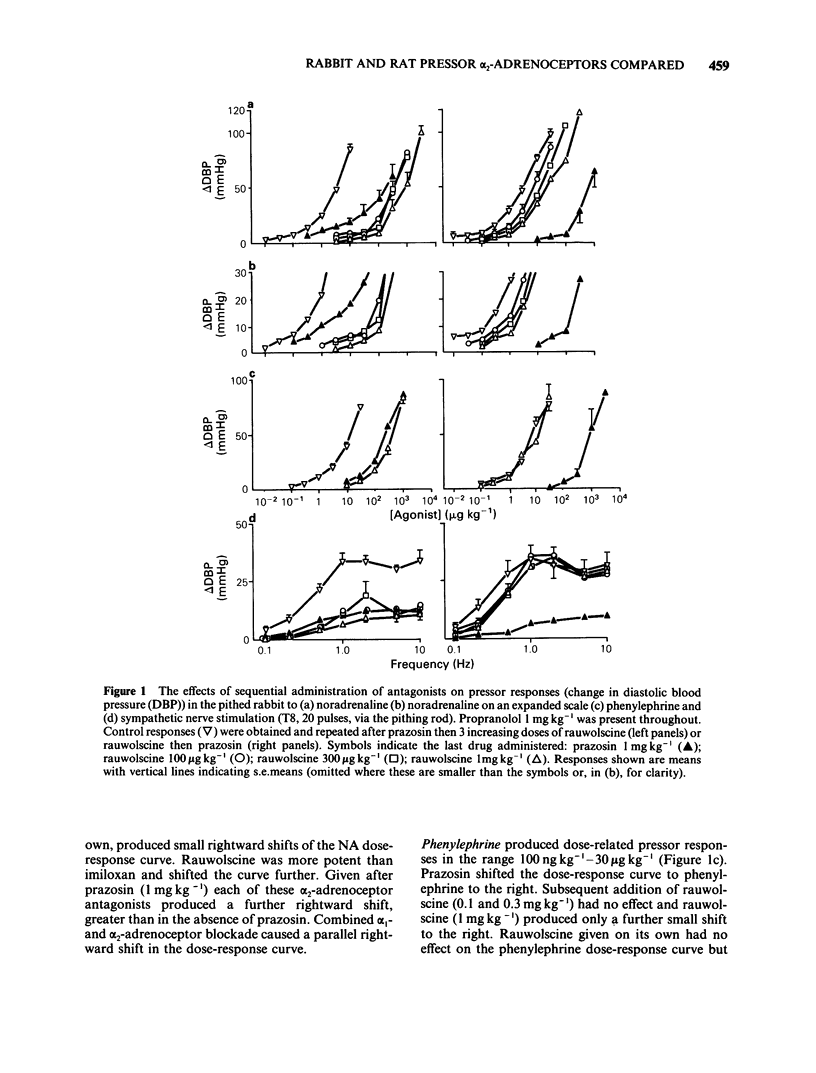
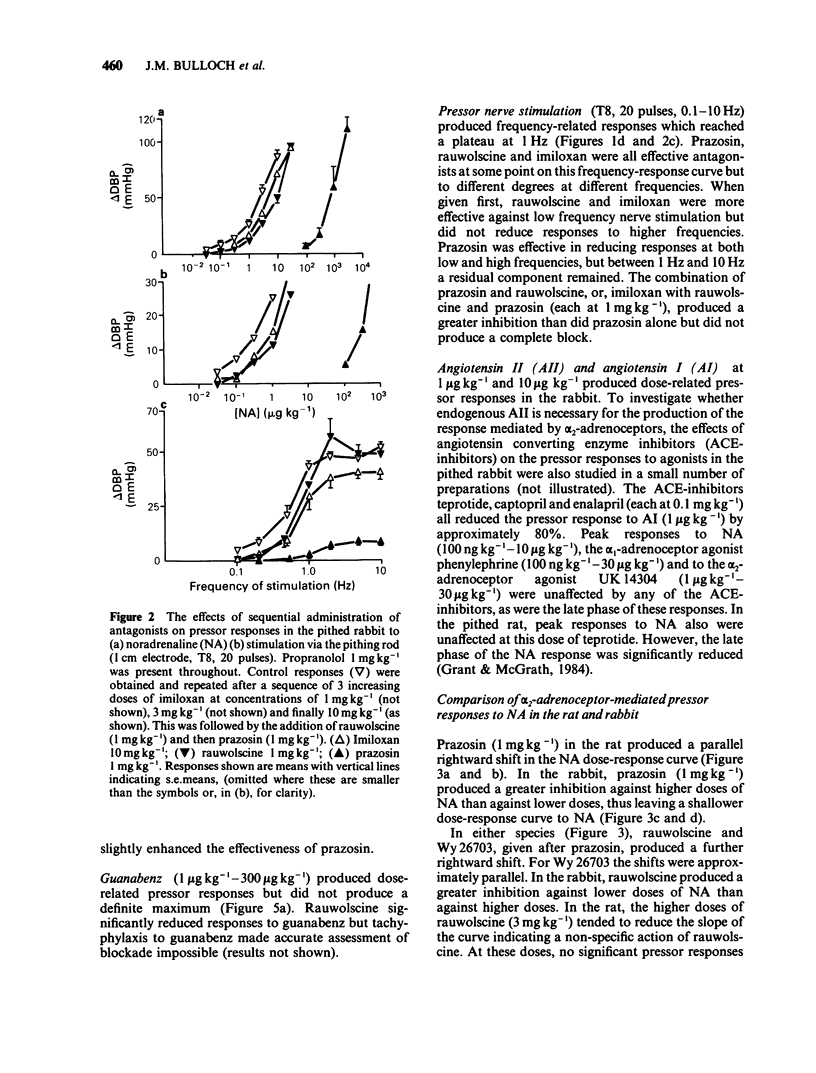
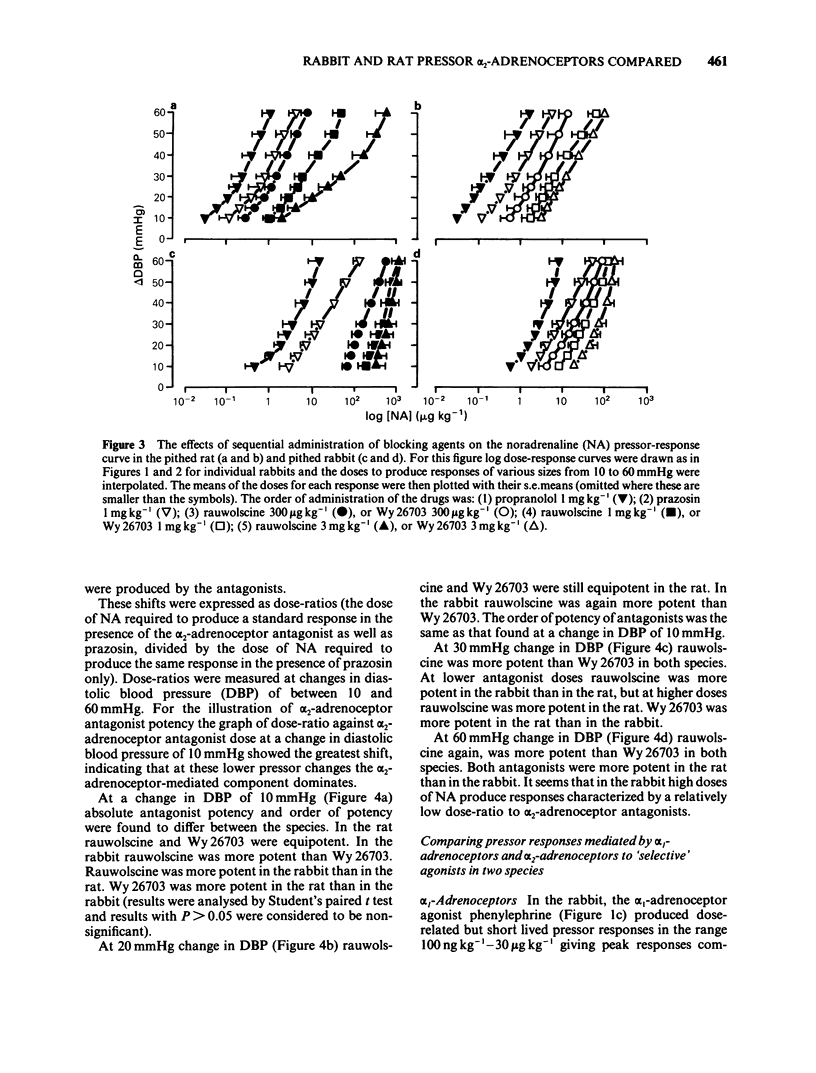
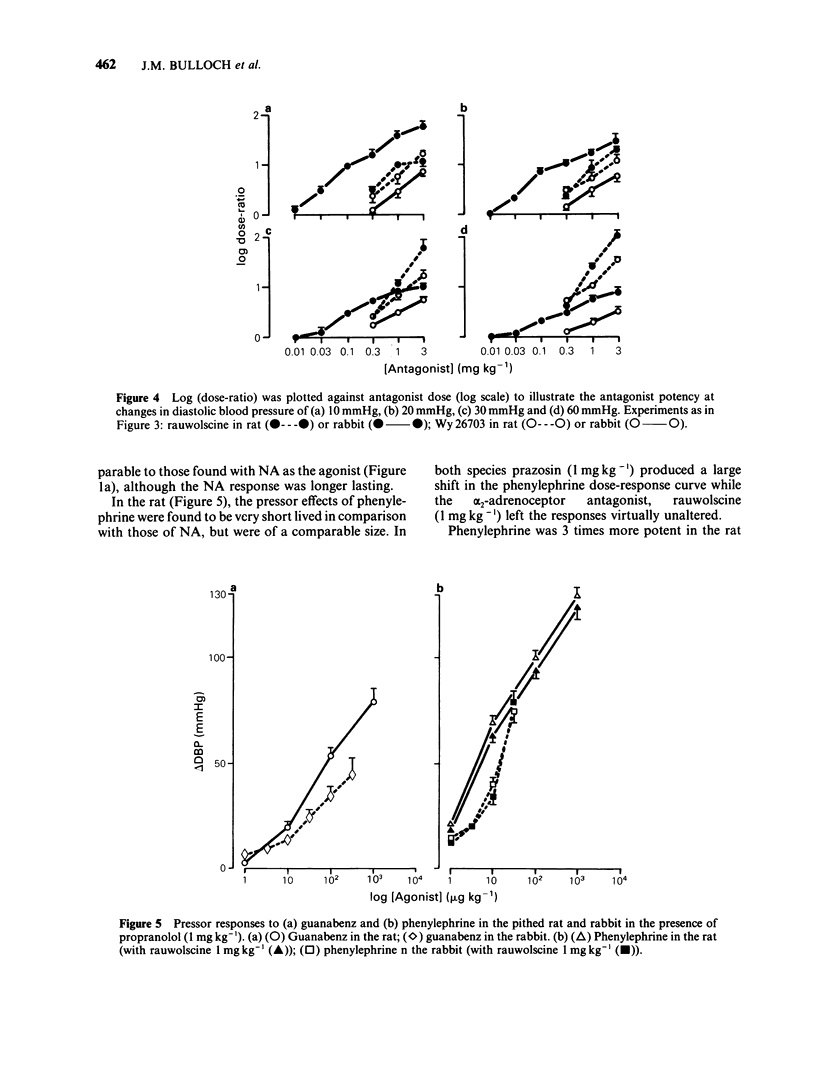
The subtypes of alpha-adrenoceptors which mediate pressor responses to sympathomimetic agonists or to nerve stimulation in pithed rabbits have been classified according to the effects of 'selective' antagonists and a comparison has been made, for the alpha 2-subtype, with corresponding responses in the rat. In the rabbit the dose-response curve for phenylephrine was shifted to the right in parallel by prazosin (1 mg kg-1) and was unaffected by rauwolscine (1 mg kg-1). The dose-response curve for noradrenaline was shifted to the right by prazosin (1 mg kg-1) and was shifted to a smaller extent by rauwolscine (1 mg kg-1) or imiloxan (10 mg kg-1). After rauwolscine, prazosin produced a rightward shift larger than when given alone. After prazosin, rauwolscine produced a rightward shift larger than when given alone. The responses to pressor nerve stimulation at low frequencies (less than 1 Hz) could be reduced by prazosin, rauwolscine or imiloxan but those at a higher frequency could be reduced only by prazosin. These results indicate that the responses to noradrenaline or to nerve stimulation are mediated by both alpha 1- and alpha 2-adrenoceptors. Low doses or frequencies have a proportionately greater component which is alpha 2. Responses to noradrenaline after prazosin (1 mg kg-1), were sufficiently sensitive to rauwolscine to be considered as predominantly alpha 2. A comparison was therefore made of such responses in the rat and rabbit. They were produced by a lower dose per unit body weight in the rat whereas this was less marked for the alpha 2-adrenoceptor agonist guanabenz. In the rabbit they were more susceptible to blockade by rauwolscine but were less sensitive to Wy 26703 than in the rat. This demonstrates that the alpha 2-adrenoceptors mediating pressor responses in vivo, like those in other tissues in vitro, are different in rat and rabbit, with regard to antagonists.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alabaster V. A., Keir R. F., Peters C. J. Comparison of activity of alpha-adrenoceptor agonists and antagonists in dog and rabbit saphenous vein. Naunyn Schmiedebergs Arch Pharmacol. 1985 Jul;330(1):33–36. doi: 10.1007/BF00586706. [DOI] [PubMed] [Google Scholar]
- Alabaster V. A., Keir R. F., Peters C. J. Comparison of potency of alpha 2-adrenoceptor antagonists in vitro: evidence for heterogeneity of alpha 2-adrenoceptors. Br J Pharmacol. 1986 Jul;88(3):607–614. doi: 10.1111/j.1476-5381.1986.tb10241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demichel P., Gomond P., Roquebert J. alpha-Adrenoceptor blocking properties of raubasine in pithed rats. Br J Pharmacol. 1982 Nov;77(3):449–454. doi: 10.1111/j.1476-5381.1982.tb09317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docherty J. R., Hyland L. No evidence for differences between pre- and post-junctional alpha 2-adrenoceptors. Br J Pharmacol. 1985 Oct;86(2):335–339. doi: 10.1111/j.1476-5381.1985.tb08901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docherty J. R., McGrath J. C. A comparison of pre- and post-junctional potencies of several alpha-adrenoceptor agonists in the cardiovascular system and anococcygeus muscle of the rat. Evidence for two types of post-junctional alpha-adrenoceptor. Naunyn Schmiedebergs Arch Pharmacol. 1980 Jun;312(2):107–116. doi: 10.1007/BF00569718. [DOI] [PubMed] [Google Scholar]
- Docherty J. R., McGrath J. C. An examination of factors influencing adrenergic transmission in the pithed rat, with special reference to noradrenaline uptake mechanisms and post-junctional alpha-adrenoceptors. Naunyn Schmiedebergs Arch Pharmacol. 1980 Aug;313(2):101–111. doi: 10.1007/BF00498564. [DOI] [PubMed] [Google Scholar]
- Drew G. M., Whiting S. B. Evidence for two distinct types of postsynaptic alpha-adrenoceptor in vascular smooth muscle in vivo. Br J Pharmacol. 1979 Oct;67(2):207–215. doi: 10.1111/j.1476-5381.1979.tb08668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavahan N. A., Grant T. L., Greig J., McGrath J. C. Analysis of the alpha-adrenoceptor-mediated, and other, components in the sympathetic vasopressor responses of the pithed rat. Br J Pharmacol. 1985 Sep;86(1):265–274. doi: 10.1111/j.1476-5381.1985.tb09458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie J. S., Maclaren A., Pollock D. A method of stimulating different segments of the autonomic outflow from the spinal column to various organs in the pithed cat and rat. Br J Pharmacol. 1970 Oct;40(2):257–267. doi: 10.1111/j.1476-5381.1970.tb09919.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant T. L., Flavahan N. A., Greig J., McGrath J. C., McKean C. E., Reid J. L. Attempts to uncover subtypes of alpha-adrenoceptors and associated mechanisms by using sequential administration of blocking drugs. Clin Sci (Lond) 1985;68 (Suppl 10):25s–30s. doi: 10.1042/cs068s025. [DOI] [PubMed] [Google Scholar]
- Hirst G. D., De Gleria S., van Helden D. F. Neuromuscular transmission in arterioles. Experientia. 1985 Jul 15;41(7):874–879. doi: 10.1007/BF01970004. [DOI] [PubMed] [Google Scholar]
- Hirst G. D., Lew M. J. Lack of involvement of alpha-adrenoceptors in sympathetic neural vasoconstriction in the hindquarters of the rabbit. Br J Pharmacol. 1987 Jan;90(1):51–60. doi: 10.1111/j.1476-5381.1987.tb16824.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer S. Z. Presynaptic regulation of catecholamine release. Biochem Pharmacol. 1974 Jul 1;23(13):1793–1800. doi: 10.1016/0006-2952(74)90187-7. [DOI] [PubMed] [Google Scholar]
- McGrath J. C. Evidence for more than one type of post-junctional alpha-adrenoceptor. Biochem Pharmacol. 1982 Feb 15;31(4):467–484. doi: 10.1016/0006-2952(82)90147-2. [DOI] [PubMed] [Google Scholar]
- McGrath J. C., Flavahan N. A., McKean C. E. alpha 1- and alpha 2-Adrenoceptor-mediated pressor and chronotropic effects in the rat and rabbit. J Cardiovasc Pharmacol. 1982;4 (Suppl 1):S101–S107. doi: 10.1097/00005344-198200041-00021. [DOI] [PubMed] [Google Scholar]
- McGrath J. C., MacKenzie J. E. The effects of intravenous anaesthetics on the cardiovascular system of the rabbit. Br J Pharmacol. 1977 Oct;61(2):199–212. doi: 10.1111/j.1476-5381.1977.tb08405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahorski S. R., Barnett D. B., Cheung Y. D. alpha-Adrenoceptor-effector coupling: affinity states or heterogeneity of the alpha 2-adrenoceptor? Clin Sci (Lond) 1985;68 (Suppl 10):39s–42s. doi: 10.1042/cs068s039. [DOI] [PubMed] [Google Scholar]
- Paciorek P. M., Shepperson N. B. alpha 1-Adrenoceptor agonist activity of alpha 2-adrenoceptor antagonists in the pithed rat preparation. Br J Pharmacol. 1983 May;79(1):12–14. doi: 10.1111/j.1476-5381.1983.tb10488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffolo R. R., Jr, Waddell J. E. Receptor interactions of imidazolines: alpha-adrenoceptors of rat and rabbit aortae differentiated by relative potencies, affinities and efficacies of imidazoline agonists. Br J Pharmacol. 1982 Sep;77(1):169–176. doi: 10.1111/j.1476-5381.1982.tb09283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schümann H. J., Lues I. Postjunctional alpha-adrenoceptors in the isolated saphenous vein of the rabbit. Characterization and influence of angiotensin. Naunyn Schmiedebergs Arch Pharmacol. 1983 Aug;323(4):328–334. doi: 10.1007/BF00512471. [DOI] [PubMed] [Google Scholar]
- Starke K. Alpha-adrenoceptor subclassification. Rev Physiol Biochem Pharmacol. 1981;88:199–236. [PubMed] [Google Scholar]
- Waterfall J. F., Rhodes K. F., Lattimer N. Studies of alpha 2-adrenoceptor antagonist potency in vitro: comparisons in tissues from rats, rabbits, dogs and humans. Clin Sci (Lond) 1985;68 (Suppl 10):21s–24s. doi: 10.1042/cs068s021. [DOI] [PubMed] [Google Scholar]


