Abstract
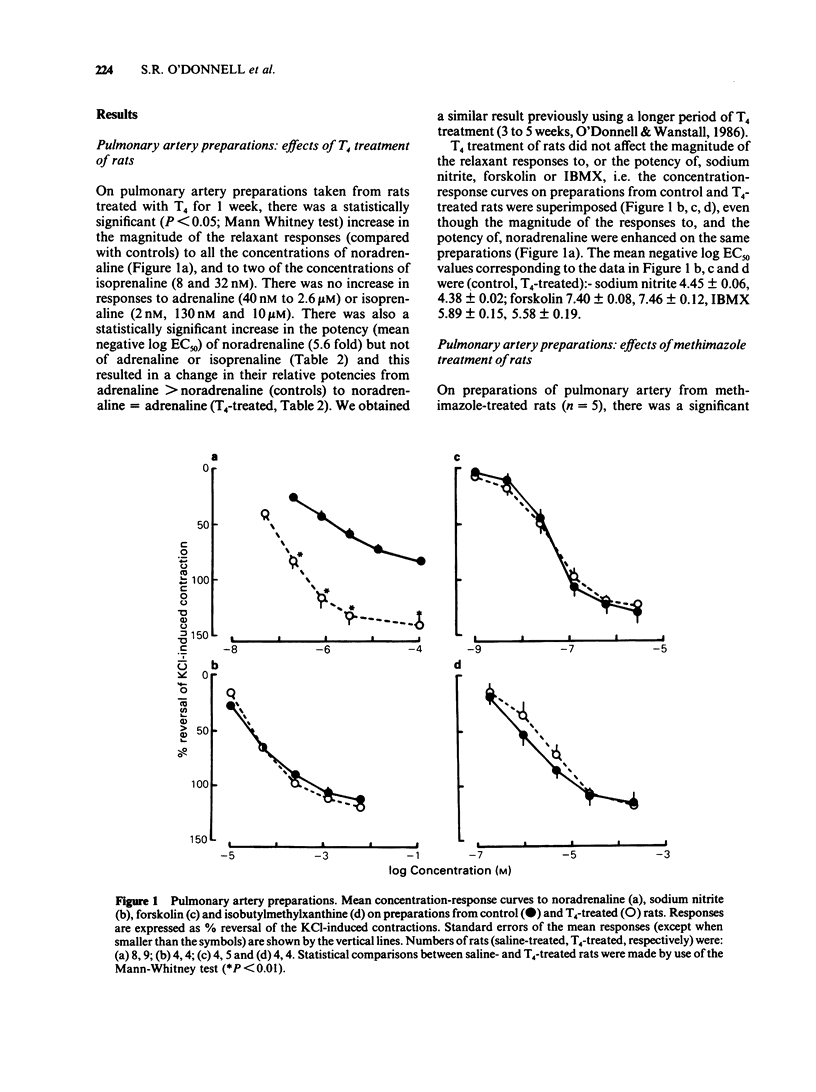
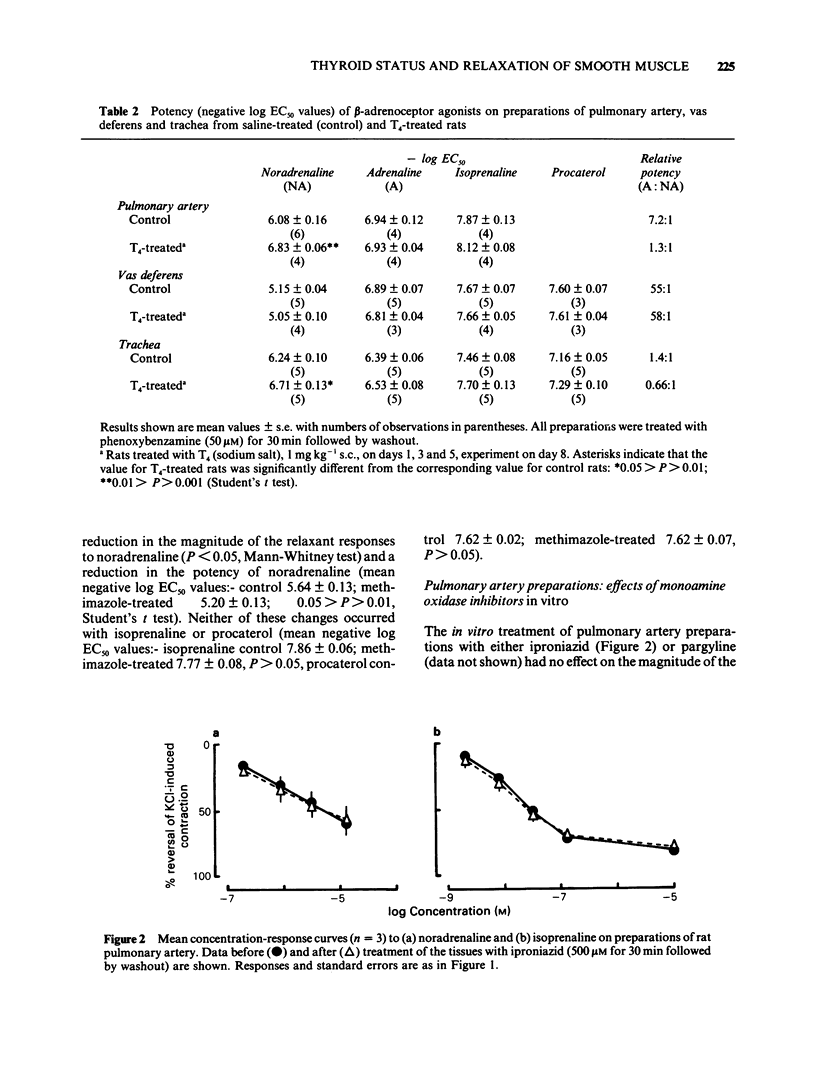
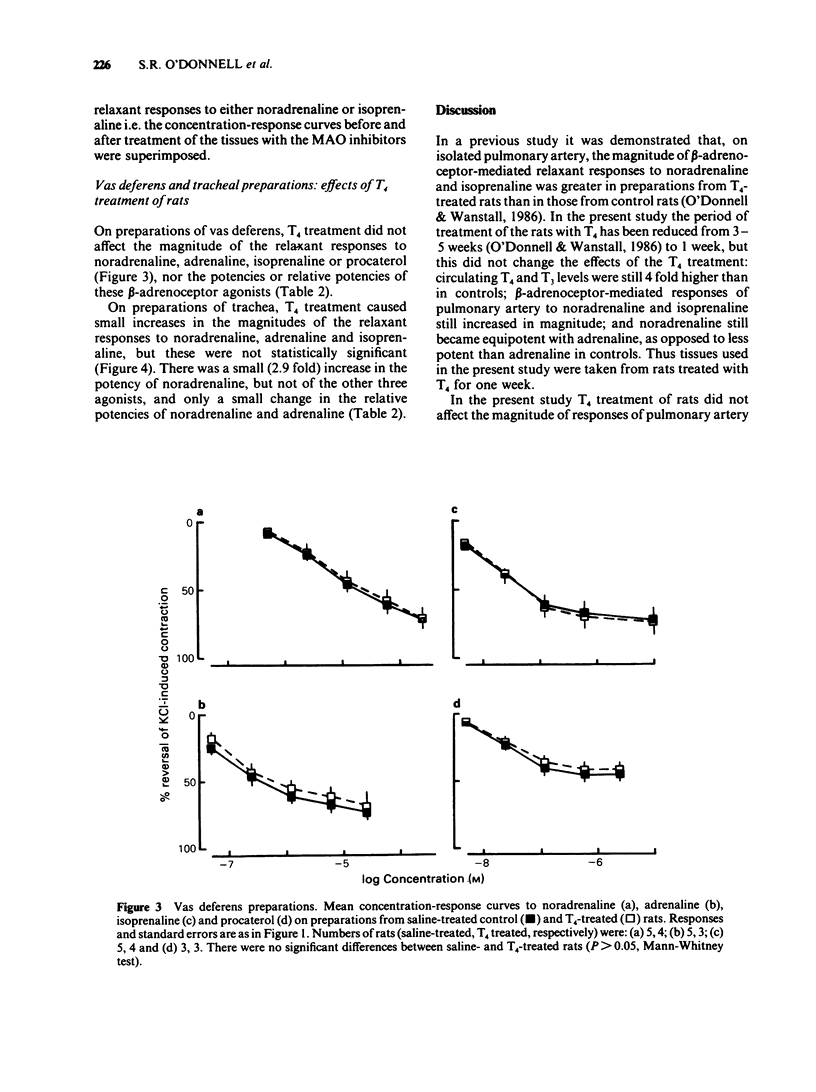
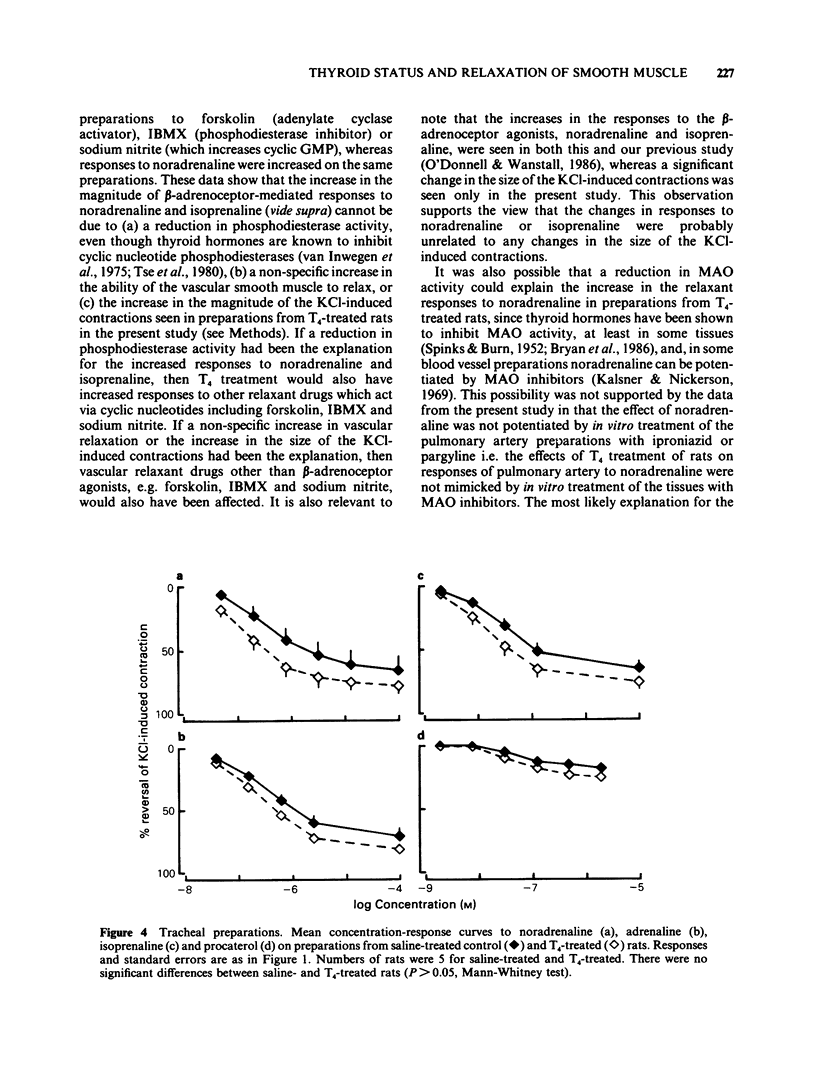
1 Responses to relaxant drugs have been examined on isolated KCl-contracted smooth muscle preparations from rats in which thyroid status was changed by prior treatment with either thyroxine (T4) for 1 week (preparations of pulmonary artery, trachea and vas deferens) or methimazole for 10-12 weeks (pulmonary artery preparations). 2 On pulmonary artery preparations, T4 treatment caused a significant increase in the magnitude of the relaxant responses to noradrenaline and isoprenaline but not those to adrenaline. The potency of noradrenaline was increased 5.6 fold but that of isoprenaline and adrenaline was unchanged. This resulted in a change in the relative potencies from adrenaline greater than noradrenaline (controls) to noradrenaline = adrenaline (T4-treated). Methimazole treatment caused a significant reduction in the magnitude of the responses to noradrenaline and in its potency (2.8 fold). Isoprenaline and procaterol were unaffected. 3 On pulmonary artery preparations, T4 treatment did not affect the magnitude of the responses to forskolin, sodium nitrite or isobutylmethylxanthine (IBMX) or their potency. In vitro treatment with the monoamine oxidase (MAO) inhibitors, iproniazid or pargyline, did not potentiate responses to either noradrenaline or isoprenaline. Therefore, it was concluded that the T4-induced changes in the magnitude of the responses to noradrenaline and isoprenaline and in the potency of noradrenaline were unlikely to be due to reduced activity of cyclic nucleotide phosphodiesterase(s) or MAO. 4 On preparations of vas deferens and trachea, T4 treatment had no effect on the magnitude of the responses to noradrenaline, isoprenaline, adrenaline or procaterol.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bryan L. J., O'Donnell S. R., Williams A. M. The effects of thyroxine treatment of rats on neuronal and extraneuronal uptake and metabolism of catecholamines in the heart. Br J Pharmacol. 1986 Feb;87(2):337–344. doi: 10.1111/j.1476-5381.1986.tb10822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox A. W., Juberg E. N., May J. M., Johnson R. D., Abel P. W., Minneman K. P. Thyroid status and adrenergic receptor subtypes in the rat: comparison of receptor density and responsiveness. J Pharmacol Exp Ther. 1985 Dec;235(3):715–723. [PubMed] [Google Scholar]
- Gross G., Lues I. Thyroid-dependent alterations of myocardial adrenoceptors and adrenoceptor-mediated responses in the rat. Naunyn Schmiedebergs Arch Pharmacol. 1985 Jun;329(4):427–439. doi: 10.1007/BF00496378. [DOI] [PubMed] [Google Scholar]
- Kalsner S., Nickerson M. Disposition of norepinephrine and epinephrine in vascular tissue, determined by the technique of oil immersion. J Pharmacol Exp Ther. 1969 Feb;165(2):152–165. [PubMed] [Google Scholar]
- Krstew E., Malta E., Raper C. Comparison of guinea pig uterine and rat vas deferens preparations for assessment of beta 2-adrenoceptor-mediated activity. J Pharmacol Methods. 1982 Dec;8(4):279–289. doi: 10.1016/0160-5402(82)90045-6. [DOI] [PubMed] [Google Scholar]
- May J. M., Abel P. W., Minneman K. P. Binding of agonists and antagonists to beta-adrenoceptors in rat vas deferens: relationship to functional response. Naunyn Schmiedebergs Arch Pharmacol. 1985 Dec;331(4):324–333. doi: 10.1007/BF00500814. [DOI] [PubMed] [Google Scholar]
- O'Donnell S. R., Wanstall J. C. Demonstration of both beta 1- and beta 2-adrenoceptors mediating relaxation of isolated ring preparations of rat pulmonary artery. Br J Pharmacol. 1981 Nov;74(3):547–552. doi: 10.1111/j.1476-5381.1981.tb10463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell S. R., Wanstall J. C. Responses to the beta 2-selective agonist procaterol of vascular and atrial preparations with different functional beta-adrenoceptor populations. Br J Pharmacol. 1985 Jan;84(1):227–235. [PMC free article] [PubMed] [Google Scholar]
- O'Donnell S. R., Wanstall J. C. Thyroxine treatment of aged or young rats demonstrates that vascular responses mediated by beta-adrenoceptor subtypes can be differentially regulated. Br J Pharmacol. 1986 May;88(1):41–49. doi: 10.1111/j.1476-5381.1986.tb09469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPINKS A., BURN J. H. Thyroid activity and amine oxidase in the liver. Br J Pharmacol Chemother. 1952 Mar;7(1):93–98. doi: 10.1111/j.1476-5381.1952.tb00694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiles G. L., Caron M. G., Lefkowitz R. J. Beta-adrenergic receptors: biochemical mechanisms of physiological regulation. Physiol Rev. 1984 Apr;64(2):661–743. doi: 10.1152/physrev.1984.64.2.661. [DOI] [PubMed] [Google Scholar]
- Stiles G. L., Lefkowitz R. J. Thyroid hormone modulation of agonist--beta-adrenergic receptor interactions in the rat heart. Life Sci. 1981 Jun 1;28(22):2529–2536. doi: 10.1016/0024-3205(81)90595-6. [DOI] [PubMed] [Google Scholar]
- Taylor S. E. Additional evidence against universal modulation of beta-adrenoceptor responses by excessive thyroxine. Br J Pharmacol. 1983 Apr;78(4):639–644. doi: 10.1111/j.1476-5381.1983.tb09414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse J., Wrenn R. W., Kuo J. F. Thyroxine-induced changes in characteristics and activities of beta-adrenergic receptors and adenosine 3',5'-monophosphate and guanosine 3',5'-monophosphate systems in the heart may be related to reputed catecholamine supersensitivity in hyperthyroidism. Endocrinology. 1980 Jul;107(1):6–16. doi: 10.1210/endo-107-1-6. [DOI] [PubMed] [Google Scholar]
- Van Inwegen R. G., Robison G. A., Thompson W. J. Cyclic nucleotide phosphodiesterases and thyroid hormones. J Biol Chem. 1975 Apr 10;250(7):2452–2456. [PubMed] [Google Scholar]
- Williams L. T., Lefkowitz R. J., Watanabe A. M., Hathaway D. R., Besch H. R., Jr Thyroid hormone regulation of beta-adrenergic receptor number. J Biol Chem. 1977 Apr 25;252(8):2787–2789. [PubMed] [Google Scholar]


