Abstract
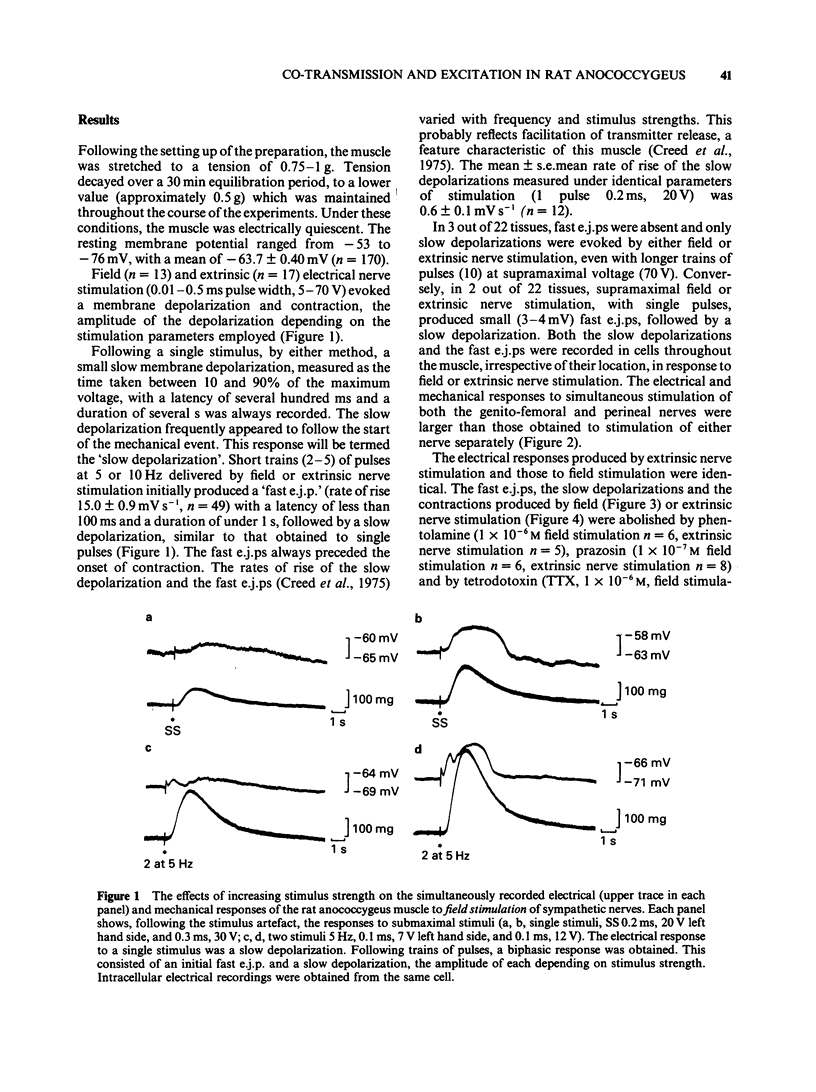
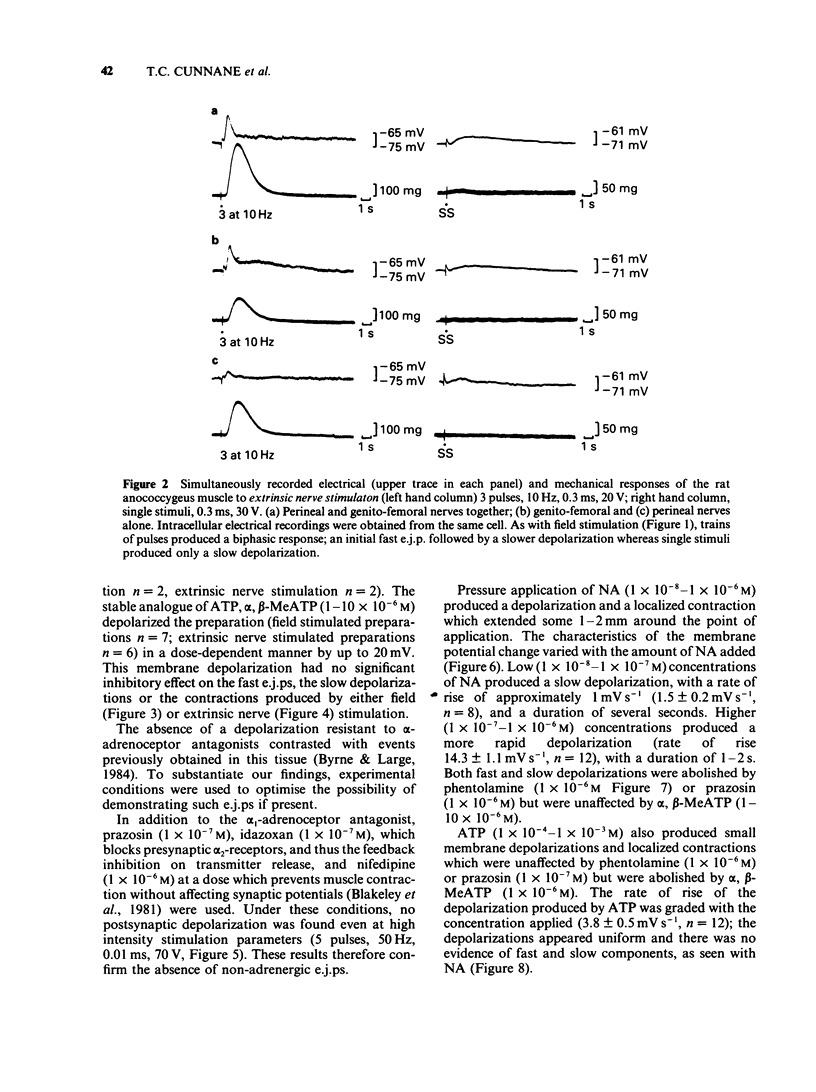
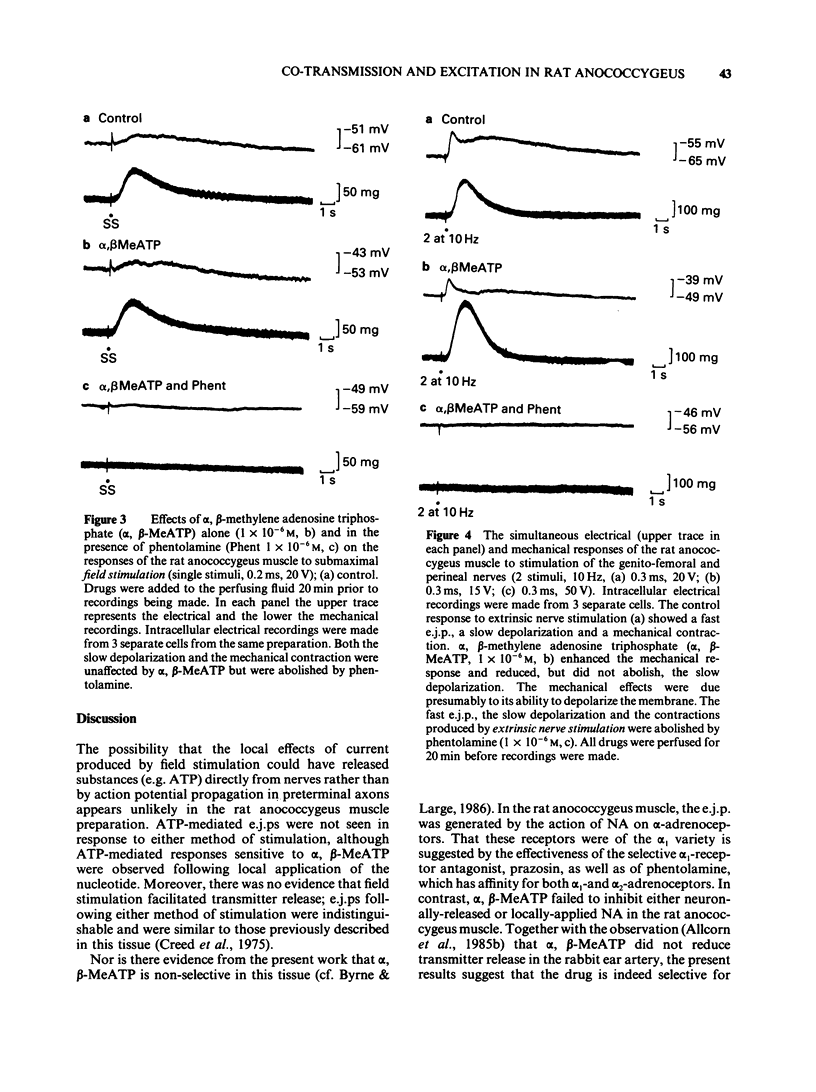
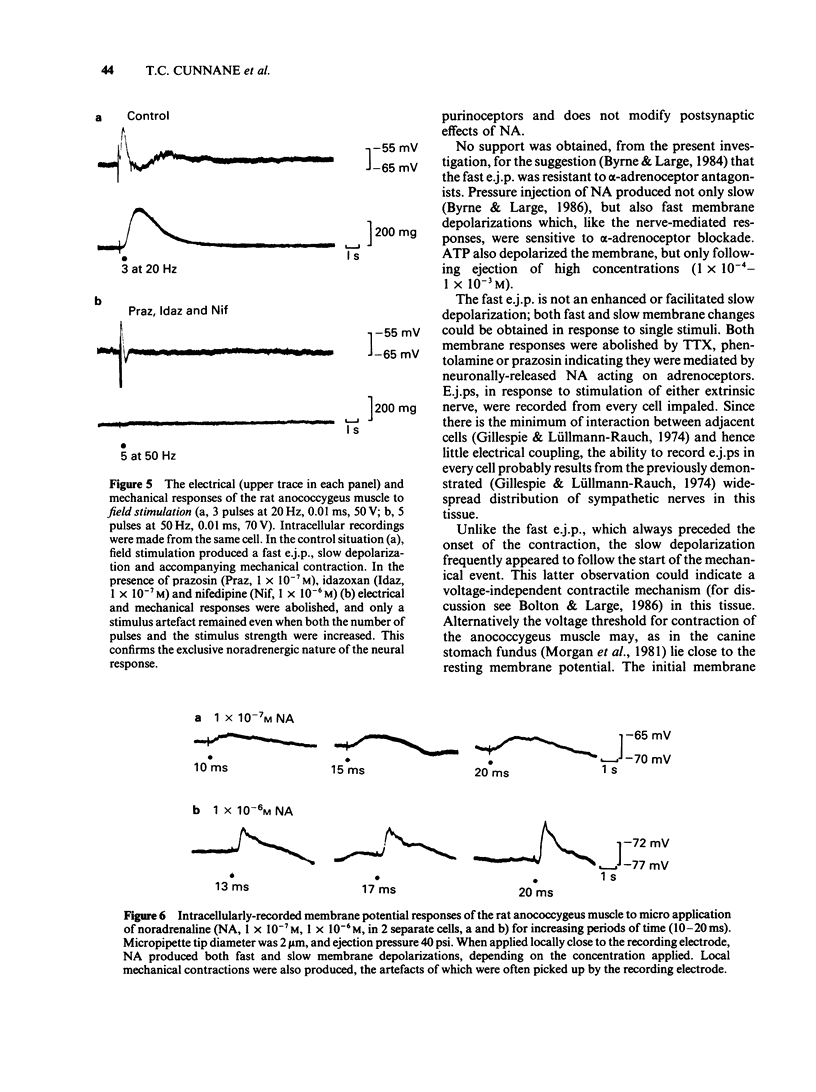
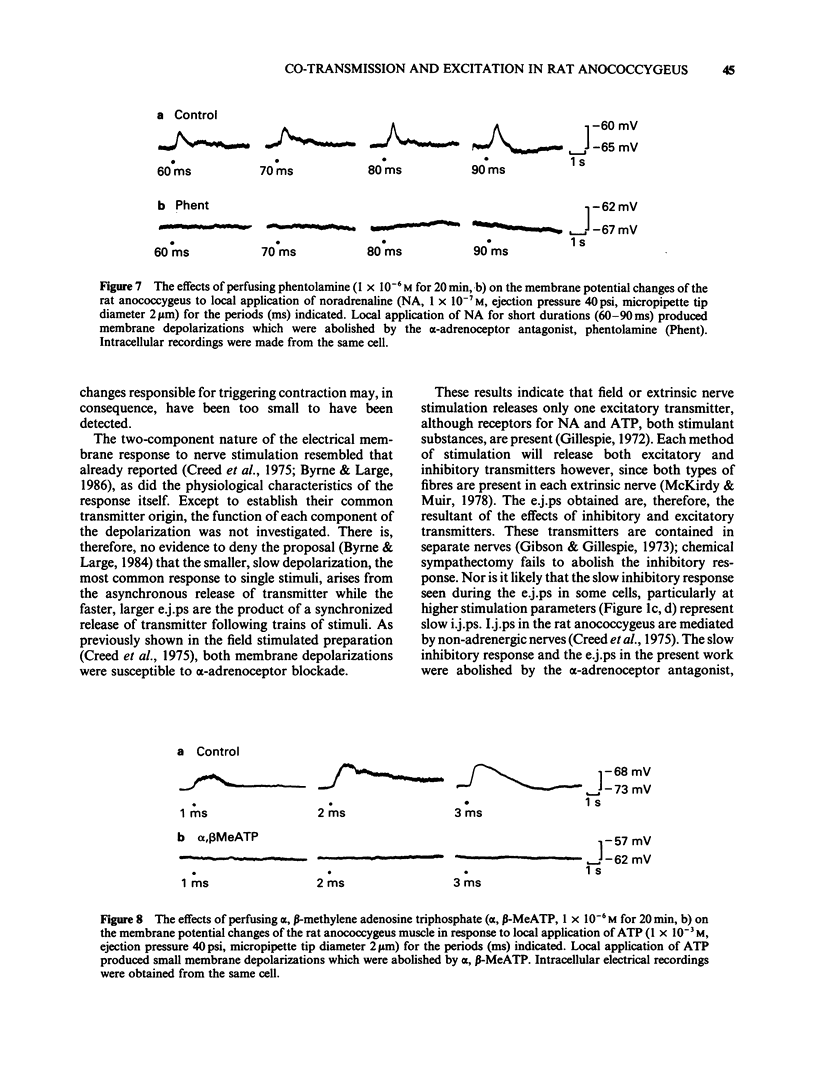
1 Electrical and mechanical responses to field (transmural) and extrinsic nerve stimulation were recorded simultaneously in the rat anococcygeus muscle. Membrane potential changes recorded intracellularly following either method of stimulation were indistinguishable. Single stimuli usually produced a slow depolarization; trains of pulses produced a fast excitatory junction potential (e.j.p.) initially, followed by a slow depolarization similar to that produced by single pulses. The fast e.j.ps, the slow depolarizations and the accompanying contractions were abolished by the alpha-adrenoceptor antagonists, phentolamine (1 X 10(-6)M) or prazosin (1 X 10(-7)M) and by tetrodotoxin (TTX, 1 X 10(-6)M) but unaffected by alpha, beta-methylene adenosine triphosphate (alpha, beta-MeATP, 1(-10) X 10(-6)M), an agent known to desensitize purinoceptors. 2 Application of noradrenaline (NA, 1 X 10(-8)-1 X 10(-6)M), by pressure ejection from a micropipette, depolarized the membrane and produced a localized contraction, both of which were abolished by phentolamine (1 X 10(-6)M) or prazosin (1 X 10(-7)M). 3 Application of adenosine-5'-triphosphate (ATP, 1 X 10(-4)-1 X 10(-3)M), by pressure ejection from a micropipette, produced a small membrane depolarization and localized contraction which were unaffected by phentolamine (1 X 10(-6)M) or prazosin (1 X 10(-7)M) but abolished by alpha, beta-MeATP (1 X 10(-6)M). 4 The results show that, in the rat annococcygeus muscle, (1) field or extrinsic nerve stimulation released only one excitatory transmitter, namely NA, although receptors for both NA and ATP were present on the muscle, (2) alpha, beta-MeATP was selective for purinoceptors and (3) there was no evidence for excitatory co-transmission in this tissue.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberts P., Bartfai T., Stjärne L. Site(s) and ionic basis of alpha-autoinhibition and facilitation of "3H'noradrenaline secretion in guinea-pig vas deferens. J Physiol. 1981 Mar;312:297–334. doi: 10.1113/jphysiol.1981.sp013630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakeley A. G., Brown D. A., Cunnane T. C., French A. M., McGrath J. C., Scott N. C. Effects of nifedipine on electrical and mechanical responses of rat and guinea pig vas deferens. Nature. 1981 Dec 24;294(5843):759–761. doi: 10.1038/294759a0. [DOI] [PubMed] [Google Scholar]
- Bolton T. B., Large W. A. Are junction potentials essential? Dual mechanism of smooth muscle cell activation by transmitter released from autonomic nerves. Q J Exp Physiol. 1986 Jan;71(1):1–28. doi: 10.1113/expphysiol.1986.sp002960. [DOI] [PubMed] [Google Scholar]
- Byrne N. G., Large W. A. Comparison of the biphasic excitatory junction potential with membrane responses to adenosine triphosphate and noradrenaline in the rat anococcygeus muscle. Br J Pharmacol. 1984 Nov;83(3):751–758. doi: 10.1111/j.1476-5381.1984.tb16229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne N. G., Large W. A. The effect of alpha, beta-methylene ATP on the depolarization evoked by noradrenaline (gamma-adrenoceptor response) and ATP in the immature rat basilar artery. Br J Pharmacol. 1986 May;88(1):6–8. doi: 10.1111/j.1476-5381.1986.tb09464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung D. W. Two components in the cellular response of rat tail arteries to nerve stimulation. J Physiol. 1982 Jul;328:461–468. doi: 10.1113/jphysiol.1982.sp014277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creed K. E., Gillespie J. S., Muir T. C. The electrical basis of excitation and inhibition in the rat anoccygeus muscle. J Physiol. 1975 Feb;245(1):33–47. doi: 10.1113/jphysiol.1975.sp010833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French A. M., Scott N. C. Evidence to support the hypothesis that ATP is a co-transmitter in rat vas deferens. Experientia. 1983 Mar 15;39(3):264–266. doi: 10.1007/BF01955295. [DOI] [PubMed] [Google Scholar]
- Gibson A., Gillespie J. S. The effect of immunosympathectomy and of 6-hydroxydopamine on the responses of the rat anococcygeus to nerve stimulation and to some drugs. Br J Pharmacol. 1973 Feb;47(2):261–267. doi: 10.1111/j.1476-5381.1973.tb08323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie J. S., Lüllmann-Rauch R. On the ultrastructure of the rat anococcygeus muscle. Cell Tissue Res. 1974;149(1):91–104. doi: 10.1007/BF00209052. [DOI] [PubMed] [Google Scholar]
- Gillespie J. S. The rat anococcygeus muscle and its response to nerve stimulation and to some drugs. Br J Pharmacol. 1972 Jul;45(3):404–416. doi: 10.1111/j.1476-5381.1972.tb08097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst G. D., Neild T. O. Localization of specialized noradrenaline receptors at neuromuscular junctions on arterioles of the guinea-pig. J Physiol. 1981;313:343–350. doi: 10.1113/jphysiol.1981.sp013669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holman M. E., Surprenant A. An electrophysiological analysis of the effects of noradrenaline and alpha-receptor antagonists on neuromuscular transmission in mammalian muscular arteries. Br J Pharmacol. 1980;71(2):651–661. doi: 10.1111/j.1476-5381.1980.tb10986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy C., Saville V. L., Burnstock G. The contributions of noradrenaline and ATP to the responses of the rabbit central ear artery to sympathetic nerve stimulation depend on the parameters of stimulation. Eur J Pharmacol. 1986 Apr 2;122(3):291–300. doi: 10.1016/0014-2999(86)90409-7. [DOI] [PubMed] [Google Scholar]
- McKirdy H. C., Muir T. C. An investigation of the role of ganglia in the innervation of the rat anococcygeus muscle: an electrical and mechanical study. Br J Pharmacol. 1978 Oct;64(2):173–184. doi: 10.1111/j.1476-5381.1978.tb17287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meldrum L. A., Burnstock G. Evidence that ATP acts as a co-transmitter with noradrenaline in sympathetic nerves supplying the guinea-pig vas deferens. Eur J Pharmacol. 1983 Aug 19;92(1-2):161–163. doi: 10.1016/0014-2999(83)90126-7. [DOI] [PubMed] [Google Scholar]
- Morgan K. G., Muir T. C., Szurszewski J. H. The electrical basis for contraction and relaxation in canine fundal smooth muscle. J Physiol. 1981 Feb;311:475–488. doi: 10.1113/jphysiol.1981.sp013599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneddon P., Burnstock G. ATP as a co-transmitter in rat tail artery. Eur J Pharmacol. 1984 Oct 30;106(1):149–152. doi: 10.1016/0014-2999(84)90688-5. [DOI] [PubMed] [Google Scholar]
- Sneddon P., Westfall D. P. Pharmacological evidence that adenosine triphosphate and noradrenaline are co-transmitters in the guinea-pig vas deferens. J Physiol. 1984 Feb;347:561–580. doi: 10.1113/jphysiol.1984.sp015083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stjärne L. Differences in secretory excitability between short and long adrenergic neurons: comparison of 3H-noradrenaline secretion evoked by field stimulation of guinea-pig vas deferens and human blood vessels. Acta Physiol Scand. 1977 Jun;100(2):264–266. doi: 10.1111/j.1748-1716.1977.tb05947.x. [DOI] [PubMed] [Google Scholar]


