Abstract
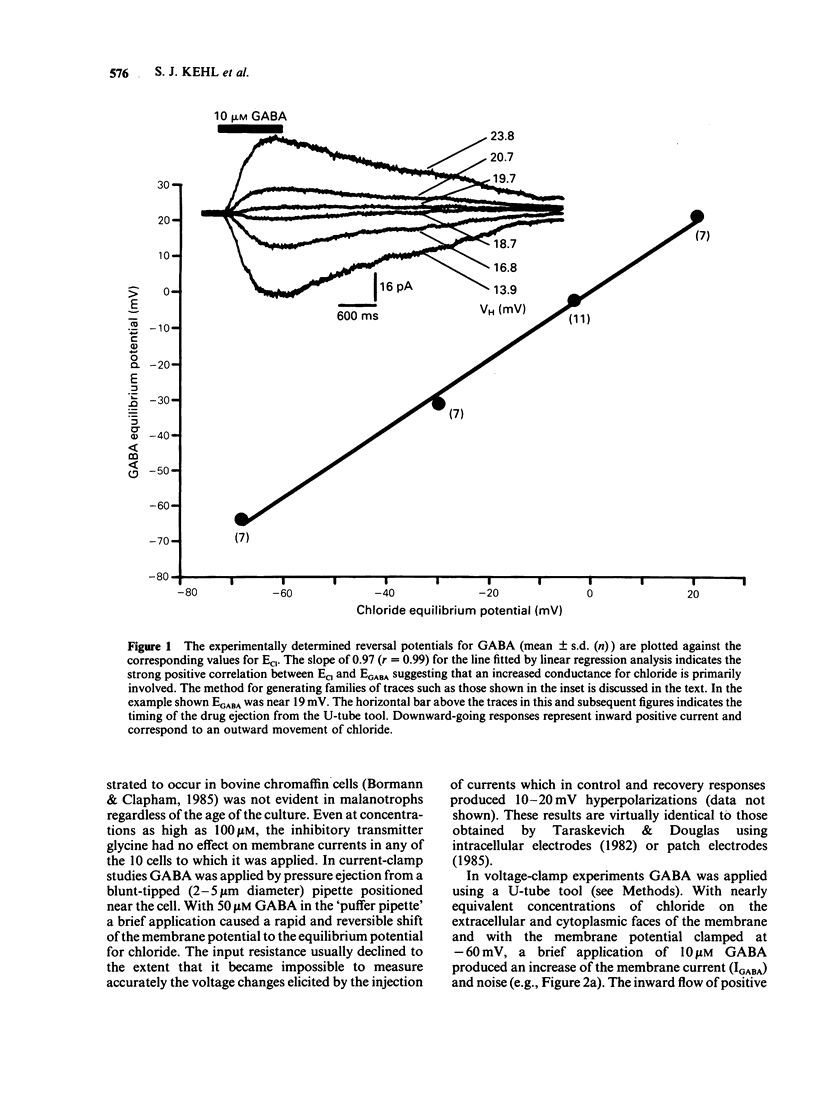
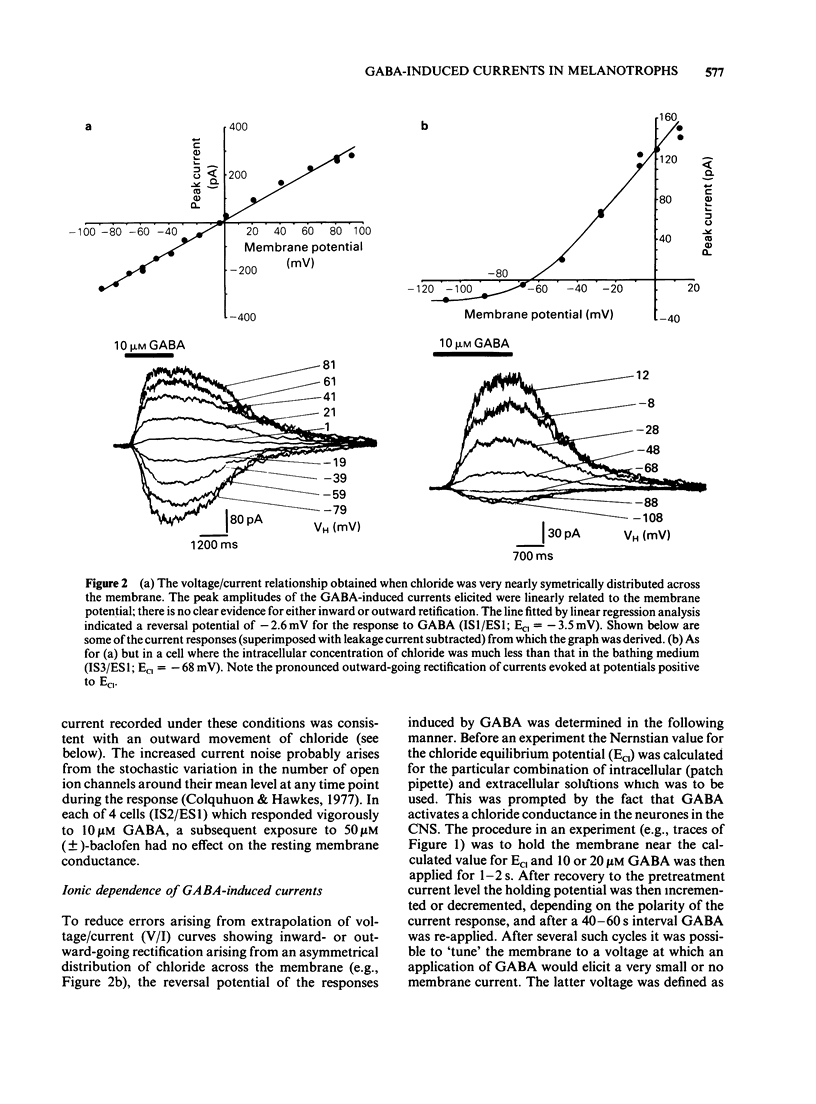
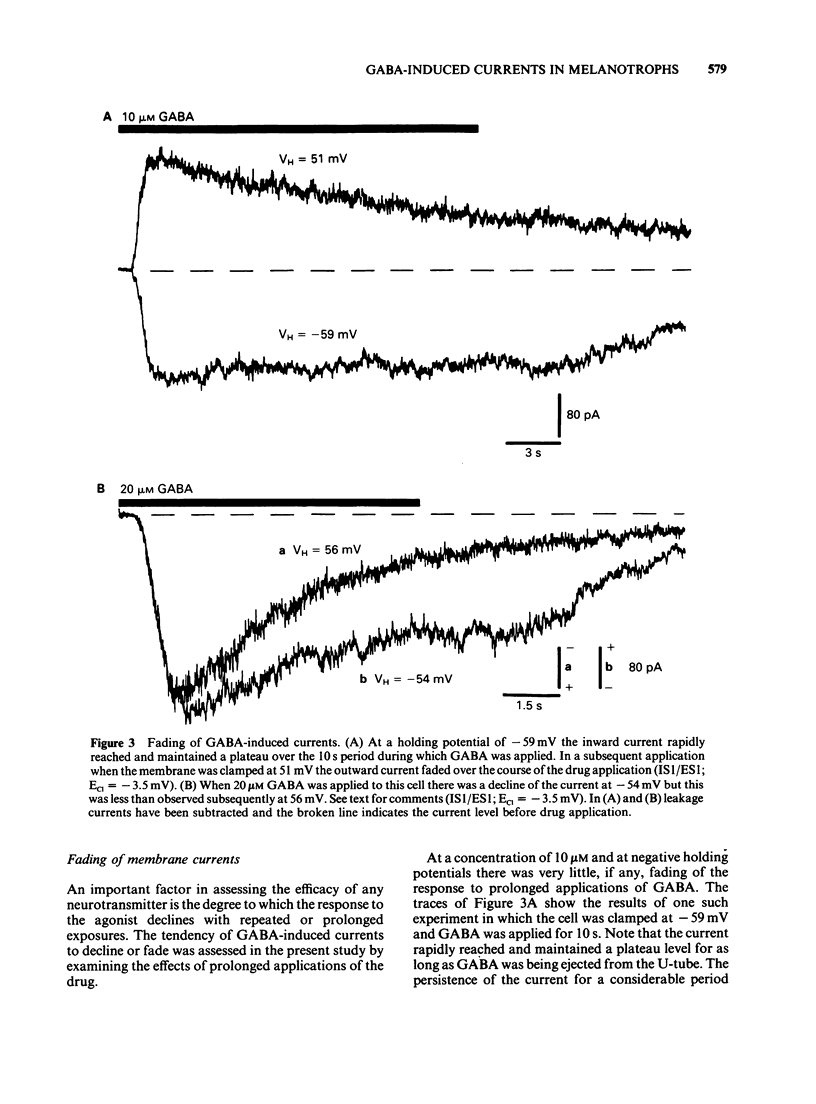
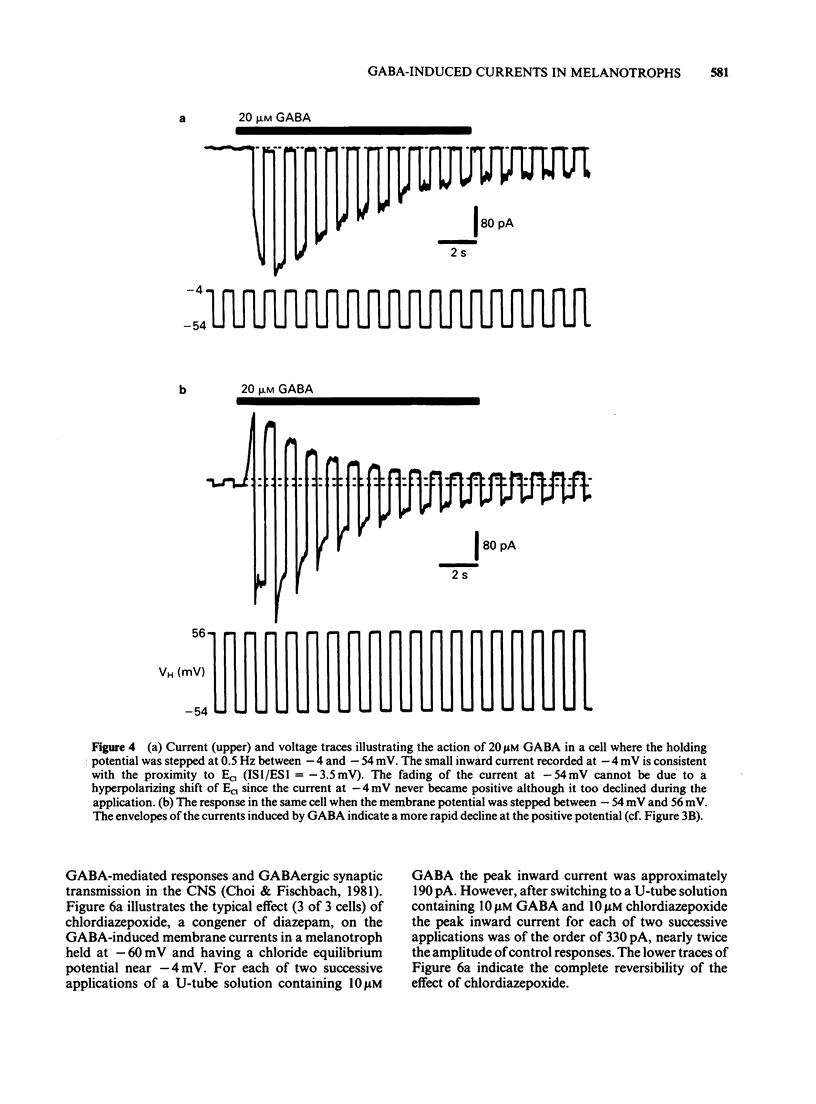
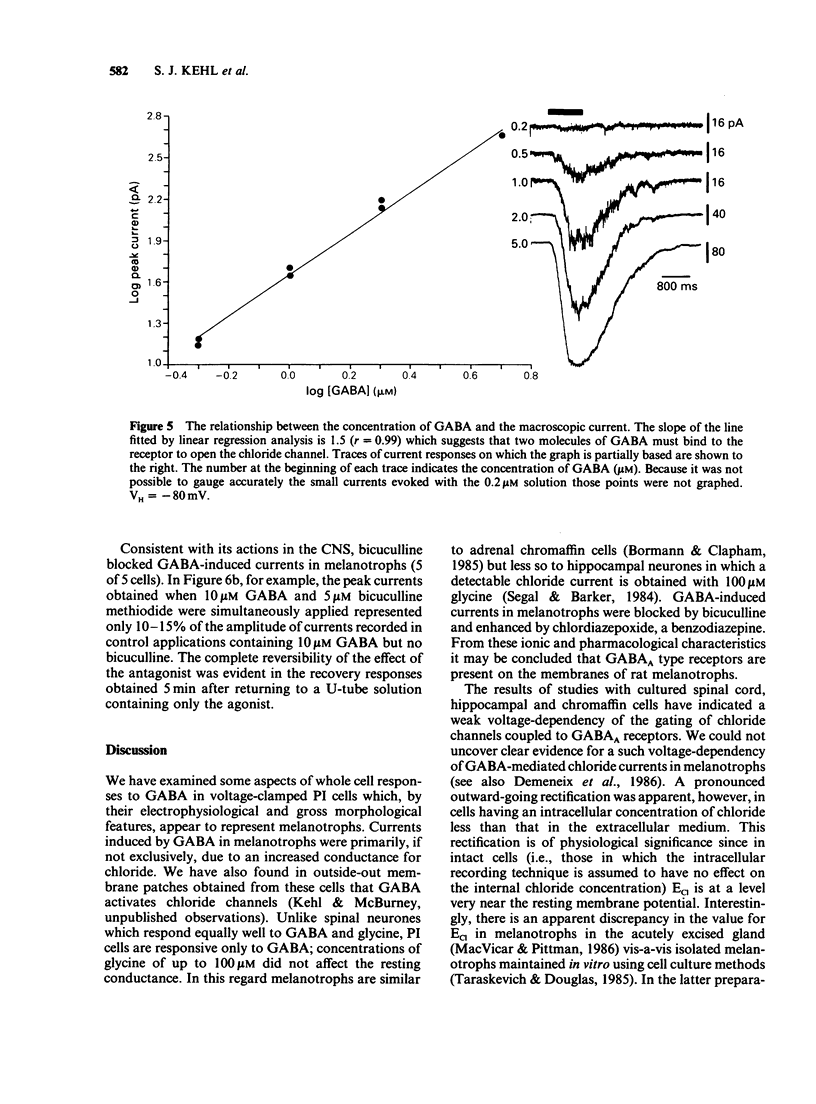
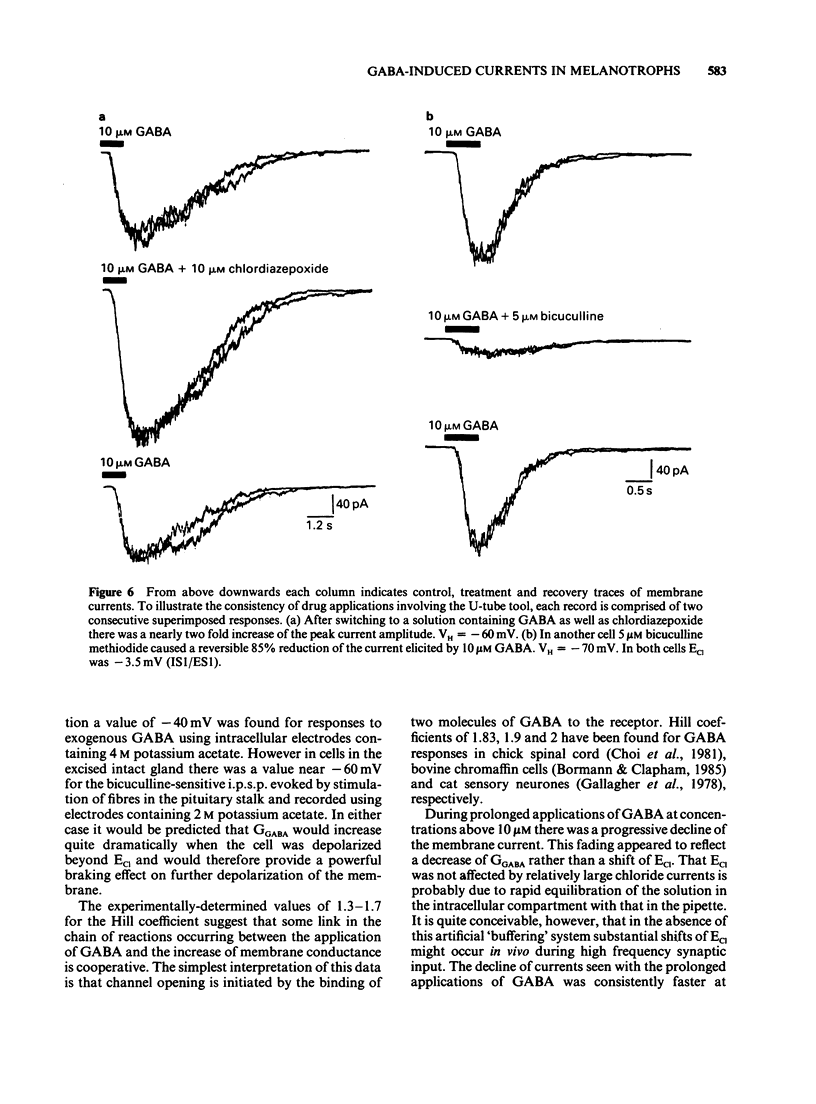
1. The macroscopic currents induced in cultured rat melanotrophs by exogenous gamma-aminobutyric acid (GABA) were analysed using the patch clamp recording technique. 2. Using various concentrations of intra- and extracellular chloride it was demonstrated that the conductance activated by GABA was chloride selective. Since these currents were blocked with bicuculline and enhanced with chlordiazepoxide the involvement of GABAA receptors similar to those in the CNS is indicated. 3. When chloride was symmetrically distributed across the membrane the voltage/current relationship was linear; pronounced rectification of GABA mediated currents was evident when there was an asymmetrical distribution of chloride. 4. With concentrations of GABA greater than 10 microM a fading of the current was seen during prolonged (5-10 s) applications. This effect appeared to be due to a decline of conductance rather than a shift of the chloride equilibrium potential. 5. Values for the Hill coefficient derived from dose-response curves suggested that the binding of 2 molecules of GABA to the receptor is required for the activation of the chloride channel. 6. There was no indication of a direct, GABAB receptor-mediated change of conductance.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R., Mitchell R. Uptake and autoreceptor-controlled release of [3H]-GABA by the hypothalamic median eminence and pituitary neurointermediate lobe. Neuroendocrinology. 1986;42(4):277–284. doi: 10.1159/000124452. [DOI] [PubMed] [Google Scholar]
- Andrade R., Malenka R. C., Nicoll R. A. A G protein couples serotonin and GABAB receptors to the same channels in hippocampus. Science. 1986 Dec 5;234(4781):1261–1265. doi: 10.1126/science.2430334. [DOI] [PubMed] [Google Scholar]
- Bormann J., Clapham D. E. gamma-Aminobutyric acid receptor channels in adrenal chromaffin cells: a patch-clamp study. Proc Natl Acad Sci U S A. 1985 Apr;82(7):2168–2172. doi: 10.1073/pnas.82.7.2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bormann J., Hamill O. P., Sakmann B. Mechanism of anion permeation through channels gated by glycine and gamma-aminobutyric acid in mouse cultured spinal neurones. J Physiol. 1987 Apr;385:243–286. doi: 10.1113/jphysiol.1987.sp016493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowery N. G., Hill D. R., Hudson A. L., Doble A., Middlemiss D. N., Shaw J., Turnbull M. (-)Baclofen decreases neurotransmitter release in the mammalian CNS by an action at a novel GABA receptor. Nature. 1980 Jan 3;283(5742):92–94. doi: 10.1038/283092a0. [DOI] [PubMed] [Google Scholar]
- Choi D. W., Farb D. H., Fischbach G. D. Chlordiazepoxide selectively potentiates GABA conductance of spinal cord and sensory neurons in cell culture. J Neurophysiol. 1981 Apr;45(4):621–631. doi: 10.1152/jn.1981.45.4.621. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Hawkes A. G. Relaxation and fluctuations of membrane currents that flow through drug-operated channels. Proc R Soc Lond B Biol Sci. 1977 Nov 14;199(1135):231–262. doi: 10.1098/rspb.1977.0137. [DOI] [PubMed] [Google Scholar]
- Demeneix B. A., Desaulles E., Feltz P., Loeffler J. P. Dual population of GABAA and GABAB receptors in rat pars intermedia demonstrated by release of alpha MSH caused by barium ions. Br J Pharmacol. 1984 May;82(1):183–190. doi: 10.1111/j.1476-5381.1984.tb16457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demeneix B. A., Taleb O., Loeffler J. P., Feltz P. GABAA and GABAB receptors on porcine pars intermedia cells in primary culture: functional role in modulating peptide release. Neuroscience. 1986 Apr;17(4):1275–1285. doi: 10.1016/0306-4522(86)90094-1. [DOI] [PubMed] [Google Scholar]
- Douglas W. W., Taraskevich P. S. Action potentials in gland cells of rat pituitary pars intermedia: inhibition by dopamine, an inhibitor of MSH secretion. J Physiol. 1978 Dec;285:171–184. doi: 10.1113/jphysiol.1978.sp012565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap K. Two types of gamma-aminobutyric acid receptor on embryonic sensory neurones. Br J Pharmacol. 1981 Nov;74(3):579–585. doi: 10.1111/j.1476-5381.1981.tb10467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. A patch-clamp study of bovine chromaffin cells and of their sensitivity to acetylcholine. J Physiol. 1982 Oct;331:577–597. doi: 10.1113/jphysiol.1982.sp014393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher J. P., Higashi H., Nishi S. Characterization and ionic basis of GABA-induced depolarizations recorded in vitro from cat primary afferent neurones. J Physiol. 1978 Feb;275:263–282. doi: 10.1113/jphysiol.1978.sp012189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray R., Johnston D. Rectification of single GABA-gated chloride channels in adult hippocampal neurons. J Neurophysiol. 1985 Jul;54(1):134–142. doi: 10.1152/jn.1985.54.1.134. [DOI] [PubMed] [Google Scholar]
- Gähwiler B. H., Brown D. A. GABAB-receptor-activated K+ current in voltage-clamped CA3 pyramidal cells in hippocampal cultures. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1558–1562. doi: 10.1073/pnas.82.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Holz G. G., 4th, Rane S. G., Dunlap K. GTP-binding proteins mediate transmitter inhibition of voltage-dependent calcium channels. Nature. 1986 Feb 20;319(6055):670–672. doi: 10.1038/319670a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe A. The mammalian pars intermedia: a review of its structure and function. J Endocrinol. 1973 Nov;59(2):385–409. doi: 10.1677/joe.0.0590385. [DOI] [PubMed] [Google Scholar]
- Huguenard J. R., Alger B. E. Whole-cell voltage-clamp study of the fading of GABA-activated currents in acutely dissociated hippocampal neurons. J Neurophysiol. 1986 Jul;56(1):1–18. doi: 10.1152/jn.1986.56.1.1. [DOI] [PubMed] [Google Scholar]
- MacVicar B. A., Pittman Q. J. Novel synaptic responses mediated by dopamine and gamma-aminobutyric acid in neuroendocrine cells of the intermediate pituitary. Neurosci Lett. 1986 Feb 14;64(1):35–40. doi: 10.1016/0304-3940(86)90659-2. [DOI] [PubMed] [Google Scholar]
- McBurney R. N., Neering I. R. The measurement of changes in intracellular free calcium during action potentials in mammalian neurones. J Neurosci Methods. 1985 Mar;13(1):65–76. doi: 10.1016/0165-0270(85)90044-5. [DOI] [PubMed] [Google Scholar]
- McCarthy K. D., de Vellis J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J Cell Biol. 1980 Jun;85(3):890–902. doi: 10.1083/jcb.85.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newberry N. R., Nicoll R. A. Direct hyperpolarizing action of baclofen on hippocampal pyramidal cells. 1984 Mar 29-Apr 4Nature. 308(5958):450–452. doi: 10.1038/308450a0. [DOI] [PubMed] [Google Scholar]
- Oertel W. H., Mugnaini E., Tappaz M. L., Weise V. K., Dahl A. L., Schmechel D. E., Kopin I. J. Central GABAergic innervation of neurointermediate pituitary lobe: biochemical and immunocytochemical study in the rat. Proc Natl Acad Sci U S A. 1982 Jan;79(2):675–679. doi: 10.1073/pnas.79.2.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinnock R. D. Hyperpolarizing action of baclofen on neurons in the rat substantia nigra slice. Brain Res. 1984 Nov 26;322(2):337–340. doi: 10.1016/0006-8993(84)90129-x. [DOI] [PubMed] [Google Scholar]
- Segal M., Barker J. L. Rat hippocampal neurons in culture: properties of GABA-activated Cl- ion conductance. J Neurophysiol. 1984 Mar;51(3):500–515. doi: 10.1152/jn.1984.51.3.500. [DOI] [PubMed] [Google Scholar]
- Taraskevich P. S., Douglas W. W. GABA directly affects electrophysiological properties of pituitary pars intermedia cells. Nature. 1982 Oct 21;299(5885):733–734. doi: 10.1038/299733a0. [DOI] [PubMed] [Google Scholar]
- Taraskevich P. S., Douglas W. W. Pharmacological and ionic features of gamma-aminobutyric acid receptors influencing electrical properties of melanotrophs isolated from the rat pars intermedia. Neuroscience. 1985 Jan;14(1):301–308. doi: 10.1016/0306-4522(85)90179-4. [DOI] [PubMed] [Google Scholar]
- Tomiko S. A., Taraskevich P. S., Douglas W. W. GABA acts directly on cells of pituitary pars intermedia to alter hormone output. Nature. 1983 Feb 24;301(5902):706–707. doi: 10.1038/301706a0. [DOI] [PubMed] [Google Scholar]
- Vincent S. R., Hökfelt T., Wu J. Y. GABA neuron systems in hypothalamus and the pituitary gland. Immunohistochemical demonstration using antibodies against glutamate decarboxylase. Neuroendocrinology. 1982 Feb;34(2):117–125. doi: 10.1159/000123288. [DOI] [PubMed] [Google Scholar]


