Abstract
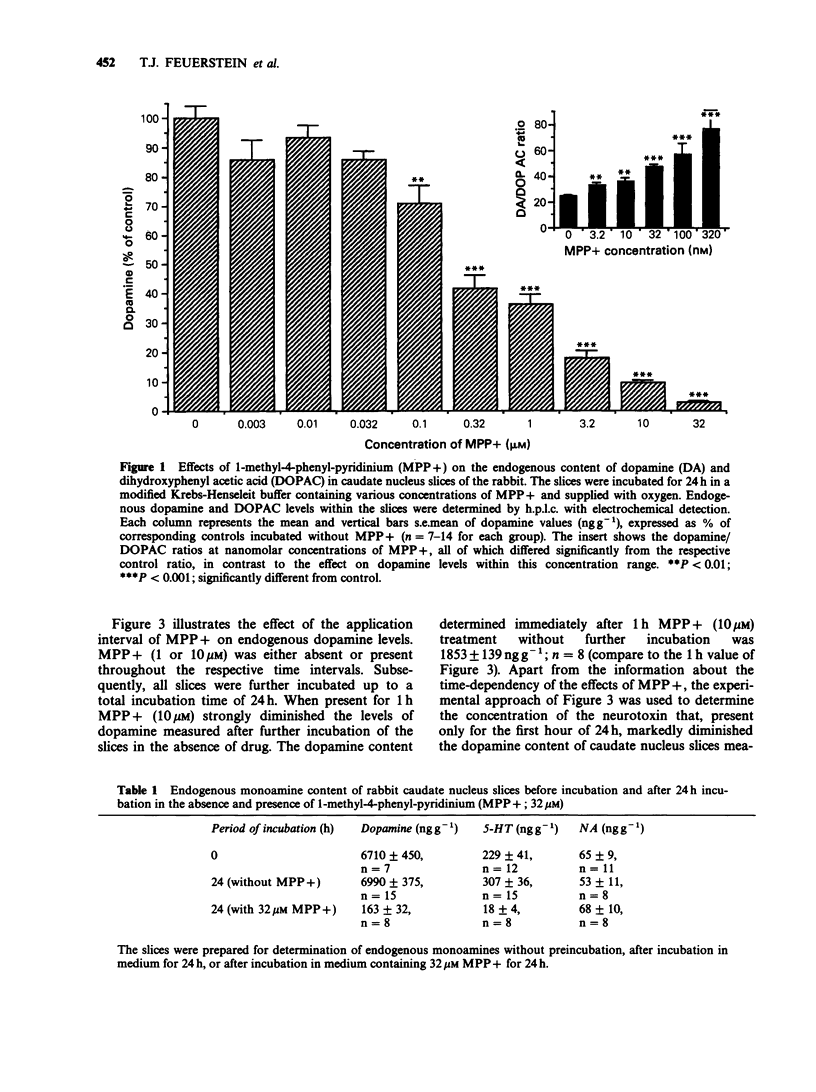
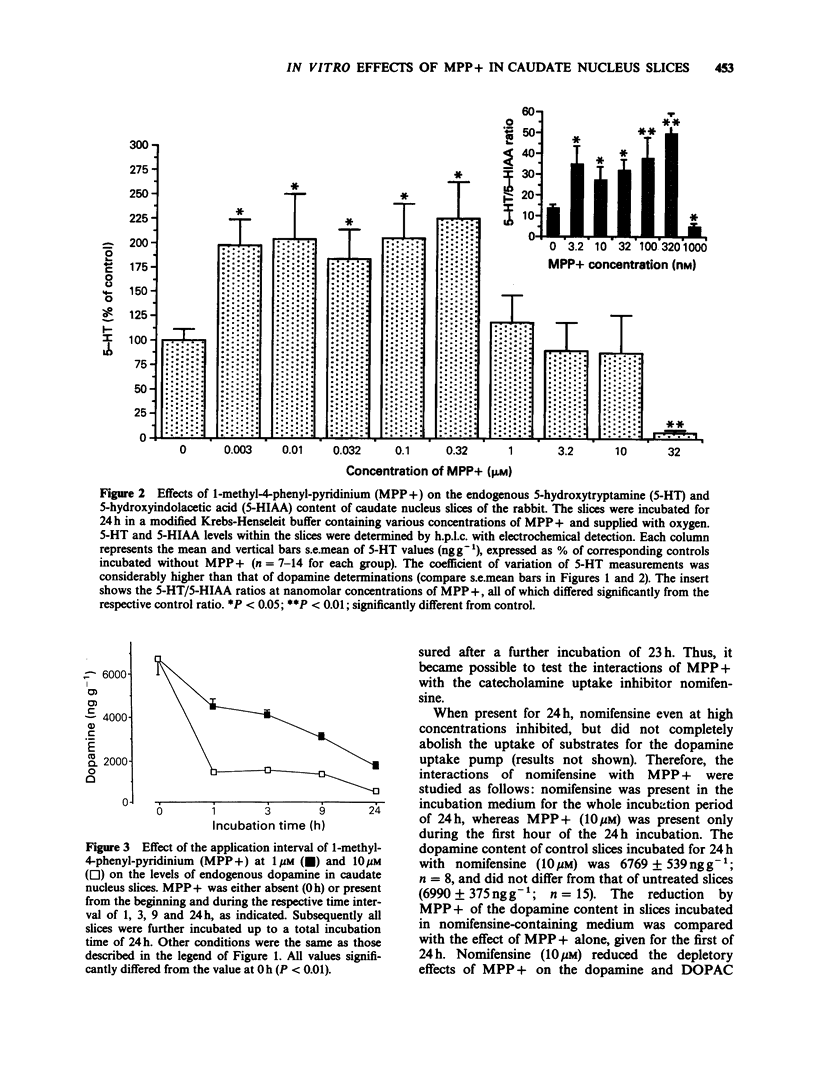
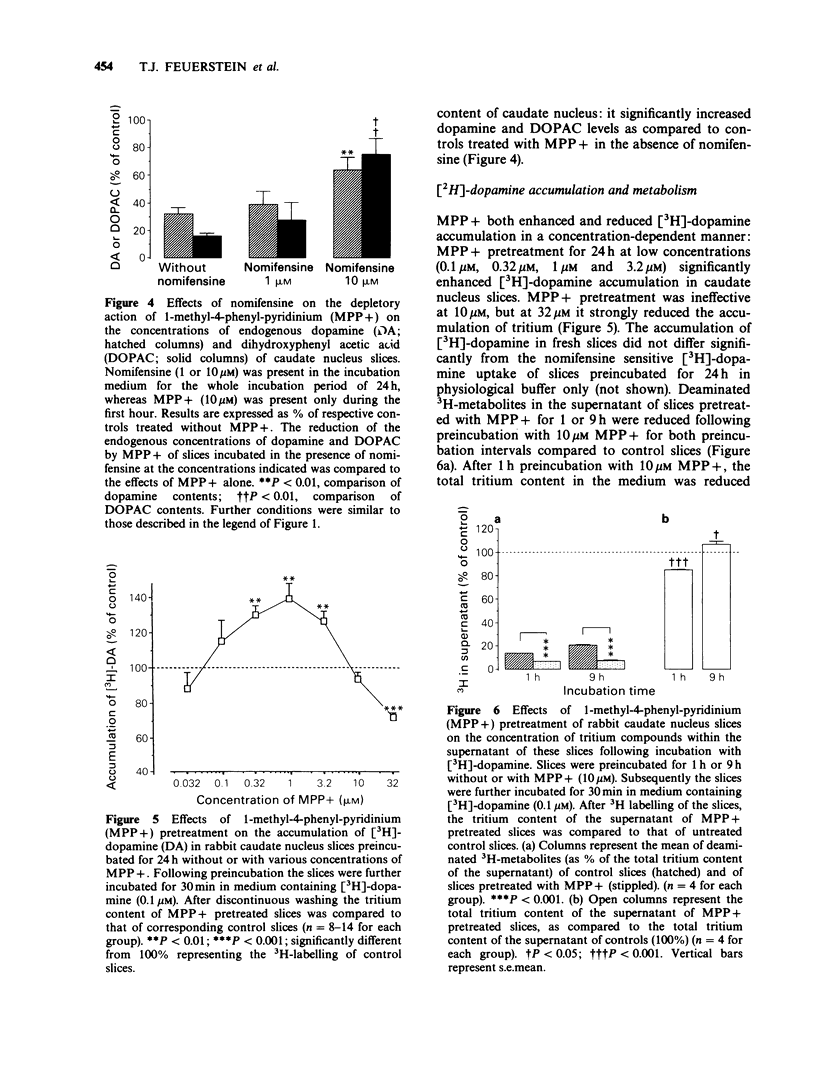
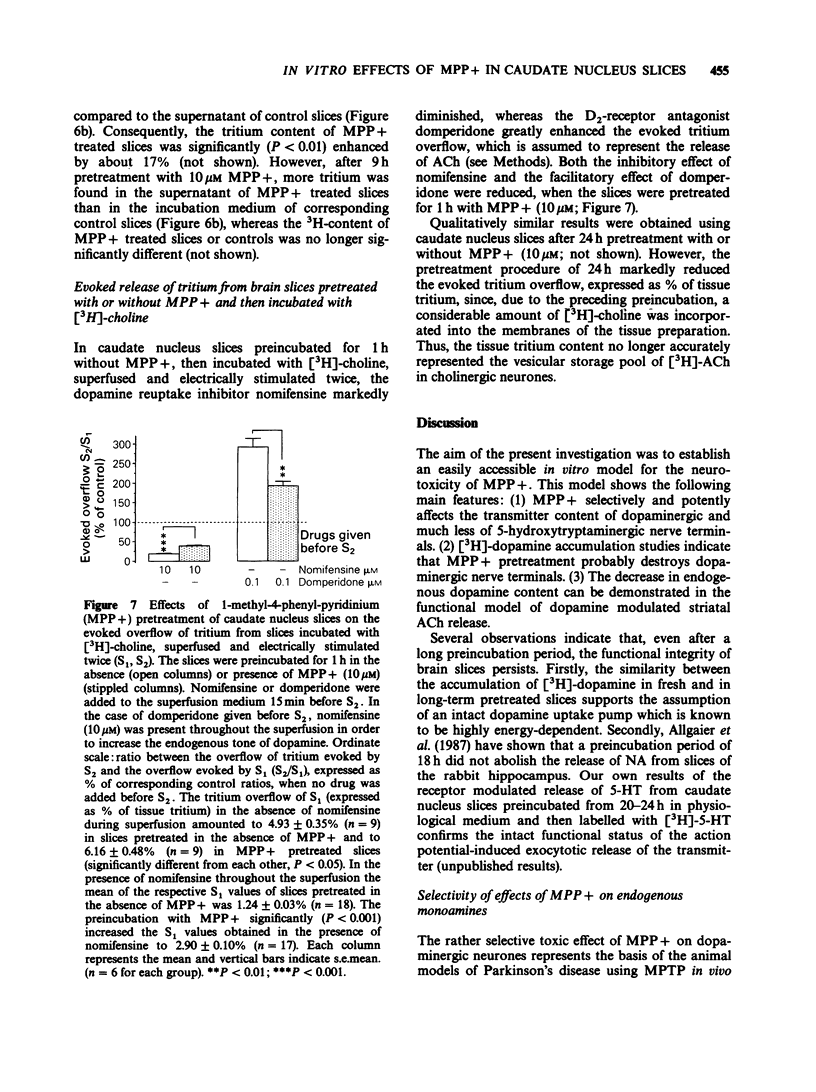
1. Slices of rabbit caudate nucleus were preincubated for up to 24 h in vitro in the presence of the neurotoxic compound 1-methyl-4-phenyl-pyridinium (MPP+). Subsequently the levels of endogenous monoamines in the slices were determined by h.p.l.c. with electrochemical detection. MPP+, in concentrations higher than 32 nM significantly diminished the dopamine levels within the slices in a concentration- and time-dependent manner; at 32 microM the depletion was more than 95%. The concentration of the major metabolite of dopamine, dihydroxyphenyl acetic acid (DOPAC) was decreased at concentrations of MPP+ that did not alter dopamine levels. Thus, MPP+ increased the dopamine/DOPAC ratio. 2. In contrast, both 5-hydroxytryptamine (5-HT) levels and 5-HT/5-hydroxyindolacetic acid (5-HIAA) ratios were increased at nanomolar concentrations of MPP+. 5-HT was significantly reduced only at 32 microM. 3. The dopamine uptake inhibitor nomifensine reduced the depletory effect of MPP+ on dopamine and DOPAC content. 4. Following 24 h pretreatment with MPP+, the uptake of [3H]-dopamine into rabbit caudate nucleus slices was either enhanced (at 0.32 microM, 1 microM and 3.2 microM MPP+) or reduced (at 32 microM MPP+). 5. Preincubation of slices with 10 microM MPP+ for only 1 h increased their 3H-labelling (in contrast to 24 h pretreatment) whereas after 9 h no net increase was detectable. After 1 and 9 h MPP+ pretreatment, much less deaminated metabolites of [3H]-dopamine were found in the incubation medium of MPP+ treated slices than in the medium of control slices. These findings suggest that MPP+ strongly inhibits the enzyme monoamine oxidase (MAO) within dopaminergic (and 5-hydroxytryptaminergic) terminals before destroying them. 6. To validate the proposed in vitro model functionally, the electrically evoked release of [3H]-acetylcholine ([3H]-ACh) was investigated in MPP+ treated slices and controls. MPP+ reduced both the facilitatory effect of the D2-receptor antagonist domperidone and the inhibitory effect of the catecholamine uptake inhibitor nomifensine on [3H]-ACh release; effects compatible with a diminished inhibitory dopaminergic input on cholinergic neurones. 7. These findings also show that the terminal region of dopaminergic neurones, the caudate nucleus, is a site for MPP+ toxicity. The present in vitro model may be useful for investigating the effects of MPP+ and its interaction with other drugs under defined conditions.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allgaier C., Hertting G., Huang H. Y., Jackisch R. Protein kinase C activation and alpha 2-autoreceptor-modulated release of noradrenaline. Br J Pharmacol. 1987 Sep;92(1):161–172. doi: 10.1111/j.1476-5381.1987.tb11308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai Y., Kinemuchi H., Hamamichi N., Satoh N., Tadano T., Kisara K. Inhibition of rat brain monoamine oxidase by some analogues of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine and 1-methyl-4-phenylpyridinium ion. Neurosci Lett. 1986 May 6;66(1):43–48. doi: 10.1016/0304-3940(86)90163-1. [DOI] [PubMed] [Google Scholar]
- Burns R. S., Chiueh C. C., Markey S. P., Ebert M. H., Jacobowitz D. M., Kopin I. J. A primate model of parkinsonism: selective destruction of dopaminergic neurons in the pars compacta of the substantia nigra by N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4546–4550. doi: 10.1073/pnas.80.14.4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba K., Trevor A. J., Castagnoli N., Jr Active uptake of MPP+, a metabolite of MPTP, by brain synaptosomes. Biochem Biophys Res Commun. 1985 May 16;128(3):1228–1232. doi: 10.1016/0006-291x(85)91071-x. [DOI] [PubMed] [Google Scholar]
- Chiba K., Trevor A., Castagnoli N., Jr Metabolism of the neurotoxic tertiary amine, MPTP, by brain monoamine oxidase. Biochem Biophys Res Commun. 1984 Apr 30;120(2):574–578. doi: 10.1016/0006-291x(84)91293-2. [DOI] [PubMed] [Google Scholar]
- Feuerstein T. J., Hertting G., Lupp A., Neufang B. False labelling of dopaminergic terminals in the rabbit caudate nucleus: uptake and release of [3H]-5-hydroxytryptamine. Br J Pharmacol. 1986 Jul;88(3):677–684. doi: 10.1111/j.1476-5381.1986.tb10250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz R. R., Abell C. W., Patel N. T., Gessner W., Brossi A. Metabolism of the neurotoxin in MPTP by human liver monoamine oxidase B. FEBS Lett. 1985 Jul 8;186(2):224–228. doi: 10.1016/0014-5793(85)80713-4. [DOI] [PubMed] [Google Scholar]
- Hertting G., Zumstein A., Jackisch R., Hoffmann I., Starke K. Modulation by endogenous dopamine of the release of acetylcholine in the caudate nucleus of the rabbit. Naunyn Schmiedebergs Arch Pharmacol. 1980;315(2):111–117. doi: 10.1007/BF00499253. [DOI] [PubMed] [Google Scholar]
- Javitch J. A., D'Amato R. J., Strittmatter S. M., Snyder S. H. Parkinsonism-inducing neurotoxin, N-methyl-4-phenyl-1,2,3,6 -tetrahydropyridine: uptake of the metabolite N-methyl-4-phenylpyridine by dopamine neurons explains selective toxicity. Proc Natl Acad Sci U S A. 1985 Apr;82(7):2173–2177. doi: 10.1073/pnas.82.7.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markstein R., Lahaye D. Neurochemical investigations in vitro with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) in preparations of rat brain. Eur J Pharmacol. 1984 Nov 13;106(2):301–311. doi: 10.1016/0014-2999(84)90717-9. [DOI] [PubMed] [Google Scholar]
- Mytilineou C., Cohen G. 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine destroys dopamine neurons in explants of rat embryo mesencephalon. Science. 1984 Aug 3;225(4661):529–531. doi: 10.1126/science.6610939. [DOI] [PubMed] [Google Scholar]
- Namura I., Douillet P., Sun C. J., Pert A., Cohen R. M., Chiueh C. C. MPP+ (1-methyl-4-phenylpyridine) is a neurotoxin to dopamine-, norepinephrine- and serotonin-containing neurons. Eur J Pharmacol. 1987 Apr 7;136(1):31–37. doi: 10.1016/0014-2999(87)90775-8. [DOI] [PubMed] [Google Scholar]
- Rollema H., Damsma G., Horn A. S., De Vries J. B., Westerink B. H. Brain dialysis in conscious rats reveals an instantaneous massive release of striatal dopamine in response to MPP+. Eur J Pharmacol. 1986 Jul 31;126(3):345–346. doi: 10.1016/0014-2999(86)90071-3. [DOI] [PubMed] [Google Scholar]
- Rollema H., De Vries J. B., Westerink B. H., Van Putten F. M., Horn A. S. Failure to detect 6-hydroxydopamine in rat striatum after the dopamine releasing drugs dexamphetamine, methylamphetamine and MPTP. Eur J Pharmacol. 1986 Dec 2;132(1):65–69. doi: 10.1016/0014-2999(86)90011-7. [DOI] [PubMed] [Google Scholar]
- Schmidt C. J., Matsuda L. A., Gibb J. W. In vitro release of tritiated monoamines from rat CNS tissue by the neurotoxic compound 1-methyl-phenyl-tetrahydropyridine. Eur J Pharmacol. 1984 Aug 17;103(3-4):255–260. doi: 10.1016/0014-2999(84)90485-0. [DOI] [PubMed] [Google Scholar]
- Singer T. P., Castagnoli N., Jr, Ramsay R. R., Trevor A. J. Biochemical events in the development of parkinsonism induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. J Neurochem. 1987 Jul;49(1):1–8. doi: 10.1111/j.1471-4159.1987.tb03384.x. [DOI] [PubMed] [Google Scholar]
- Snyder S. H., D'Amato R. J. MPTP: a neurotoxin relevant to the pathophysiology of Parkinson's disease. The 1985 George C. Cotzias lecture. Neurology. 1986 Feb;36(2):250–258. doi: 10.1212/wnl.36.2.250. [DOI] [PubMed] [Google Scholar]
- Starke K., Hedler L., Steppeler A. Metabolism of endogenous and exogenous noradrenaline in guinea-pig atria. Naunyn Schmiedebergs Arch Pharmacol. 1981 Nov;317(3):193–198. doi: 10.1007/BF00503815. [DOI] [PubMed] [Google Scholar]
- Steppeler A., Döring C., Hedler L., Starke K. Effect of amezinium on the release and catabolism of 3H-monoamines in brain slices. Biochem Pharmacol. 1982 Jul 15;31(14):2395–2402. doi: 10.1016/0006-2952(82)90535-4. [DOI] [PubMed] [Google Scholar]


