Abstract
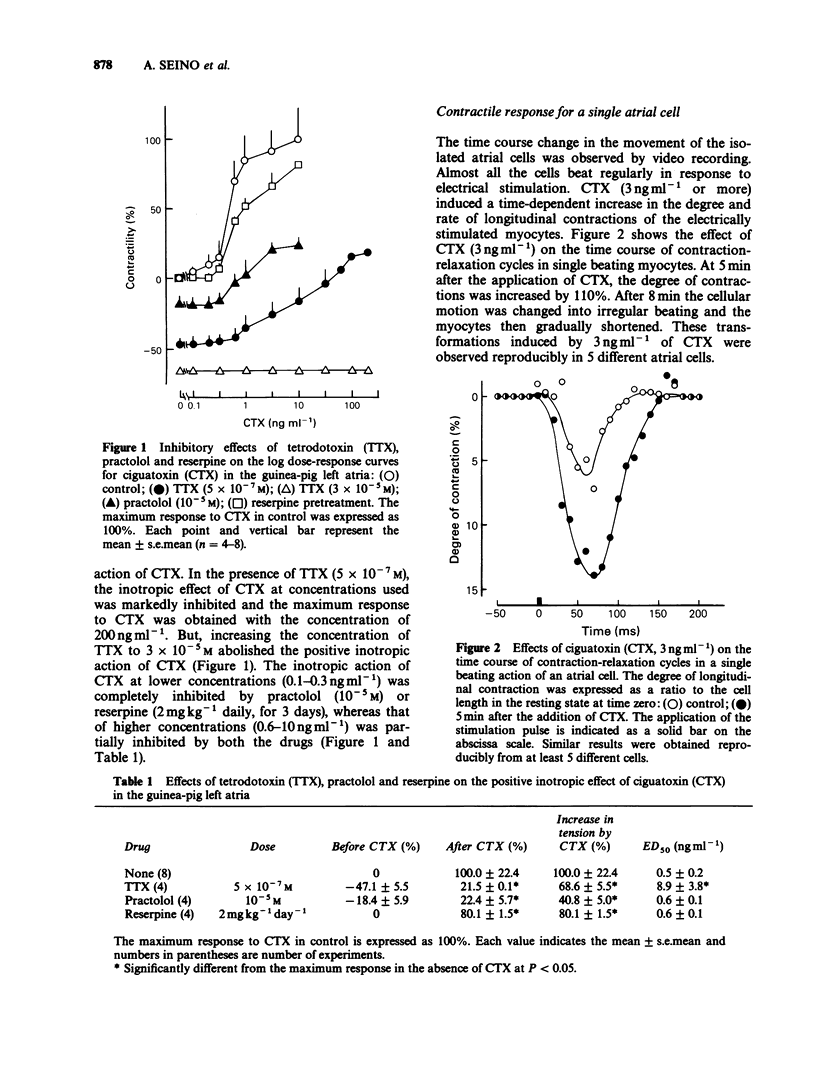
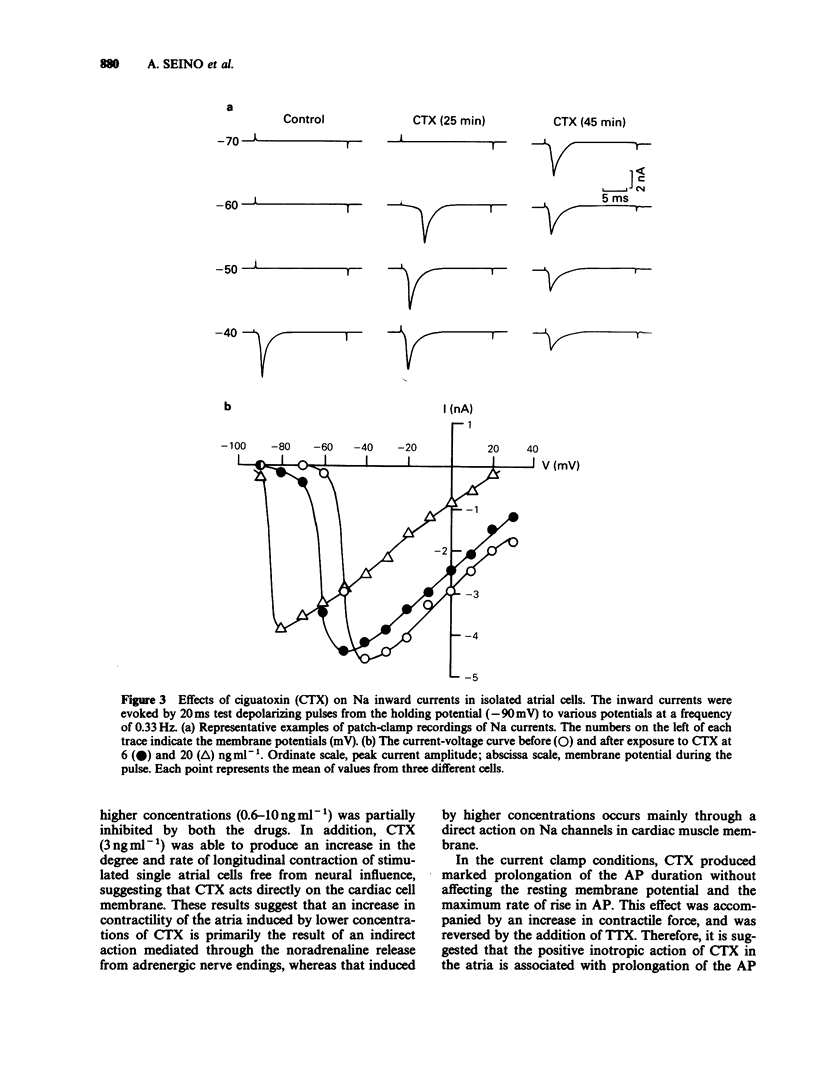
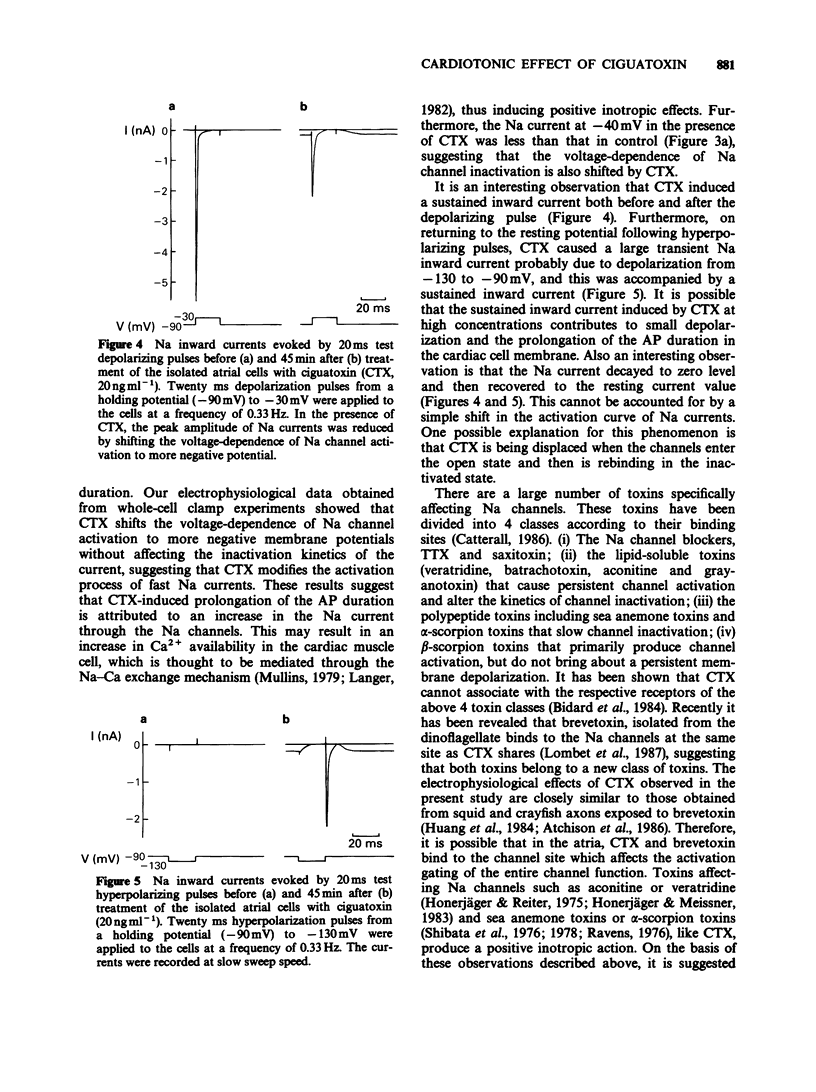
1. Ciguatoxin (CTX) caused a dose-dependent increase in the contractile force of the guinea-pig isolated left atria at concentrations ranging from 0.1 to 10 ng ml-1 with the ED50 value of 0.5 ng ml-1. 2. In the atria, tetrodotoxin (5 x 10(-7) M) inhibited markedly the inotropic action of CTX. The inotropic effect of CTX at low concentrations was abolished by practolol (10(-5) M) and reserpine (2 mg kg-1 daily, for 3 days), whereas that of CTX at high concentrations was partially inhibited by both drugs. 3. In single atrial cells, CTX (3 ng ml-1) produced a marked increase in the amplitude of longitudinal contractions. 4. CTX (3 ng ml-1) caused marked prolongation in the falling phase of action potentials of atrial strips without affecting the maximum rate of rise of action potentials and membrane resting potentials. The effect of CTX on action potentials was abolished by tetrodotoxin (10(-6) M). 5. The whole-cell patch-clamp experiments on myocytes revealed that CTX (20 ng ml-1) shifted the current-voltage curve of Na inward currents by 40 mV in the negative direction. CTX caused a small sustained Na inward current even at resting membrane potentials. 6. These results suggest that the inotropic action of lower concentrations of CTX is primarily due to an indirect action via noradrenaline release, whereas that of higher concentrations is caused not only by an indirect action but also by a direct action on voltage-dependent Na channels of cardiac muscle.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atchison W. D., Luke V. S., Narahashi T., Vogel S. M. Nerve membrane sodium channels as the target site of brevetoxins at neuromuscular junctions. Br J Pharmacol. 1986 Dec;89(4):731–738. doi: 10.1111/j.1476-5381.1986.tb11177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer M., Best P. M., Reuter H. Voltage-dependent action of tetrodotoxin in mammalian cardiac muscle. Nature. 1976 Sep 23;263(5575):344–345. doi: 10.1038/263344a0. [DOI] [PubMed] [Google Scholar]
- Bidard J. N., Vijverberg H. P., Frelin C., Chungue E., Legrand A. M., Bagnis R., Lazdunski M. Ciguatoxin is a novel type of Na+ channel toxin. J Biol Chem. 1984 Jul 10;259(13):8353–8357. [PubMed] [Google Scholar]
- Catterall W. A. Molecular properties of voltage-sensitive sodium channels. Annu Rev Biochem. 1986;55:953–985. doi: 10.1146/annurev.bi.55.070186.004513. [DOI] [PubMed] [Google Scholar]
- Gillespie N. C., Lewis R. J., Pearn J. H., Bourke A. T., Holmes M. J., Bourke J. B., Shields W. J. Ciguatera in Australia. Occurrence, clinical features, pathophysiology and management. Med J Aust. 1986 Dec 1;145(11-12):584–590. [PubMed] [Google Scholar]
- Honerjäger P., Meissner A. The positive inotropic effect of aconitine. Naunyn Schmiedebergs Arch Pharmacol. 1983 Feb;322(1):49–58. doi: 10.1007/BF00649352. [DOI] [PubMed] [Google Scholar]
- Honerjäger P., Reiter M. The relation between the effects of veratridine on action potential and contraction in mammalian ventricular myocardium. Naunyn Schmiedebergs Arch Pharmacol. 1975;289(1):1–28. doi: 10.1007/BF00498026. [DOI] [PubMed] [Google Scholar]
- Huang J. M., Wu C. H., Baden D. G. Depolarizing action of a red-tide dinoflagellate brevetoxin on axonal membranes. J Pharmacol Exp Ther. 1984 May;229(2):615–621. [PubMed] [Google Scholar]
- Kobayashi M., Kondo S., Yasumoto T., Ohizumi Y. Cardiotoxic effects of maitotoxin, a principal toxin of seafood poisoning, on guinea pig and rat cardiac muscle. J Pharmacol Exp Ther. 1986 Sep;238(3):1077–1083. [PubMed] [Google Scholar]
- Kobayashi M., Ochi R., Ohizumi Y. Maitotoxin-activated single calcium channels in guinea-pig cardiac cells. Br J Pharmacol. 1987 Nov;92(3):665–671. doi: 10.1111/j.1476-5381.1987.tb11370.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer G. A. Sodium-calcium exchange in the heart. Annu Rev Physiol. 1982;44:435–449. doi: 10.1146/annurev.ph.44.030182.002251. [DOI] [PubMed] [Google Scholar]
- Lewis R. J., Endean R. Direct and indirect effects of ciguatoxin on guinea-pig atria and papillary muscles. Naunyn Schmiedebergs Arch Pharmacol. 1986 Nov;334(3):313–322. doi: 10.1007/BF00508787. [DOI] [PubMed] [Google Scholar]
- Lewis R. J., Endean R. Mode of action of ciguatoxin from the Spanish Mackerel, Scomberomorus commersoni, on the guinea-pig ileum and vas deferens. J Pharmacol Exp Ther. 1984 Mar;228(3):756–760. [PubMed] [Google Scholar]
- Lombet A., Bidard J. N., Lazdunski M. Ciguatoxin and brevetoxins share a common receptor site on the neuronal voltage-dependent Na+ channel. FEBS Lett. 1987 Jul 27;219(2):355–359. doi: 10.1016/0014-5793(87)80252-1. [DOI] [PubMed] [Google Scholar]
- Miyahara J. T., Akau C. K., Yasumoto T. Effects of ciguatoxin and maitotoxin on the isolated guinea pig atria. Res Commun Chem Pathol Pharmacol. 1979 Jul;25(1):177–180. [PubMed] [Google Scholar]
- Mullins L. J. The generation of electric currents in cardiac fibers by Na/Ca exchange. Am J Physiol. 1979 Mar;236(3):C103–C110. doi: 10.1152/ajpcell.1979.236.3.C103. [DOI] [PubMed] [Google Scholar]
- Narahashi T. Mechanism of action of tetrodotoxin and saxitoxin on excitable membranes. Fed Proc. 1972 May-Jun;31(3):1124–1132. [PubMed] [Google Scholar]
- Nukina M., Koyanagi L. M., Scheuer P. J. Two interchangeable forms of ciguatoxin. Toxicon. 1984;22(2):169–176. doi: 10.1016/0041-0101(84)90017-5. [DOI] [PubMed] [Google Scholar]
- Ohizumi Y., Ishida Y., Shibata S. Mode of the ciguatoxin-induced supersensitivity in the guinea-pig vas deferens. J Pharmacol Exp Ther. 1982 Jun;221(3):748–752. [PubMed] [Google Scholar]
- Ohizumi Y., Kobayashi M., Muroyama A., Nakamura H., Kobayashi J. The mechanism of the inotropic action of striatoxin, a novel polypeptide toxin from a marine snail, in isolated cardiac muscle. Br J Pharmacol. 1988 Nov;95(3):867–875. doi: 10.1111/j.1476-5381.1988.tb11716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohizumi Y., Shibata S., Tachibana K. Mode of the excitatory and inhibitory actions of ciguatoxin in the guinea-pig vas deferens. J Pharmacol Exp Ther. 1981 May;217(2):475–480. [PubMed] [Google Scholar]
- Ohshika H. Marine toxins from the Pacific. IX. Some effects of ciguatoxin on isolated mammalian atria. Toxicon. 1971 Oct;9(4):337–343. doi: 10.1016/0041-0101(71)90131-0. [DOI] [PubMed] [Google Scholar]
- Ravens U. Electromechanical studies of an Anemonia sulcata toxin in mammalian cardiac muscle. Naunyn Schmiedebergs Arch Pharmacol. 1976 Dec;296(1):73–78. doi: 10.1007/BF00498842. [DOI] [PubMed] [Google Scholar]
- Rayner M. D. Mode of action of ciguatoxin. Fed Proc. 1972 May-Jun;31(3):1139–1145. [PubMed] [Google Scholar]
- Scheuer P. J., Takahashi W., Tsutsumi J., Yoshida T. Ciguatoxin: isolation and chemical nature. Science. 1967 Mar 10;155(3767):1267–1268. doi: 10.1126/science.155.3767.1267. [DOI] [PubMed] [Google Scholar]
- Setliff J. A., Rayner M. D., Hong S. K. Effect of ciguatoxin on sodium transport across the frog skin. Toxicol Appl Pharmacol. 1971 Mar;18(3):676–684. doi: 10.1016/s0041-008x(71)80022-4. [DOI] [PubMed] [Google Scholar]
- Shibata S., Izumi T., Seriguchi D. G., Norton T. R. Further studies on the positive inotropic effect of the polypeptide anthopleurin-A from a sea anemone. J Pharmacol Exp Ther. 1978 Jun;205(3):683–692. [PubMed] [Google Scholar]
- Shibata S., Norton T. R., Izumi T., Matsuo T., Katsuki S. A polypeptide (AP-A) from sea anemone (Anthopleura xanthogrammica) with potent positive inotropic action. J Pharmacol Exp Ther. 1976 Nov;199(2):298–309. [PubMed] [Google Scholar]


