Abstract
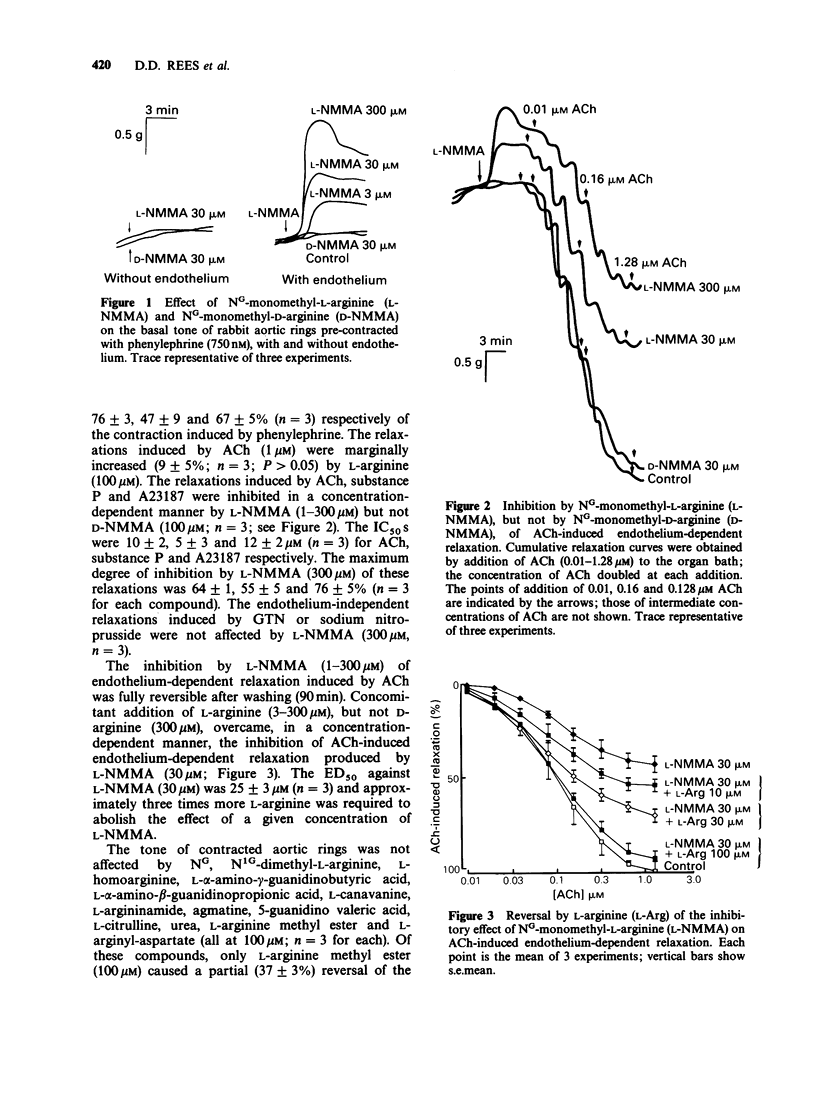
1. The role of L-arginine in the basal and stimulated generation of nitric oxide (NO) for endothelium-dependent relaxation was studied by use of NG-monomethyl L-arginine (L-NMMA), a specific inhibitor of this pathway. 2. L-Arginine (10-100 microM), but not D-arginine (100 microM), induced small but significant endothelium-dependent relaxations of rings of rabbit aorta. In contrast, L-NMMA (1-300 microM) produced small, endothelium-dependent contractions, while its enantiomer NG-monomethyl-D-arginine (D-NMMA; 100 microM) had no effect. 3. L-NMMA (1-300 microM) inhibited endothelium-dependent relaxations induced by acetylcholine (ACh), the calcium ionophore A23187, substance P or L-arginine without affecting the endothelium-independent relaxations induced by glyceryl trinitrate or sodium nitroprusside. 4. The inhibition of endothelium-dependent relaxation by L-NMMA (30 microM) was reversed by L-arginine (3-300 microM) but not by D-arginine (300 microM) or a number of close analogues (100 microM). 5. The release of NO induced by ACh from perfused segments of rabbit aorta was also inhibited by L-NMMA (3-300 microM), but not by D-NMMA (100 microM) and this effect of L-NMMA was reversed by L-arginine (3-300 microM). 6. These results support the proposal that L-arginine is the physiological precursor for the basal and stimulated generation of NO for endothelium-dependent relaxation.
Full text
PDF




Selected References
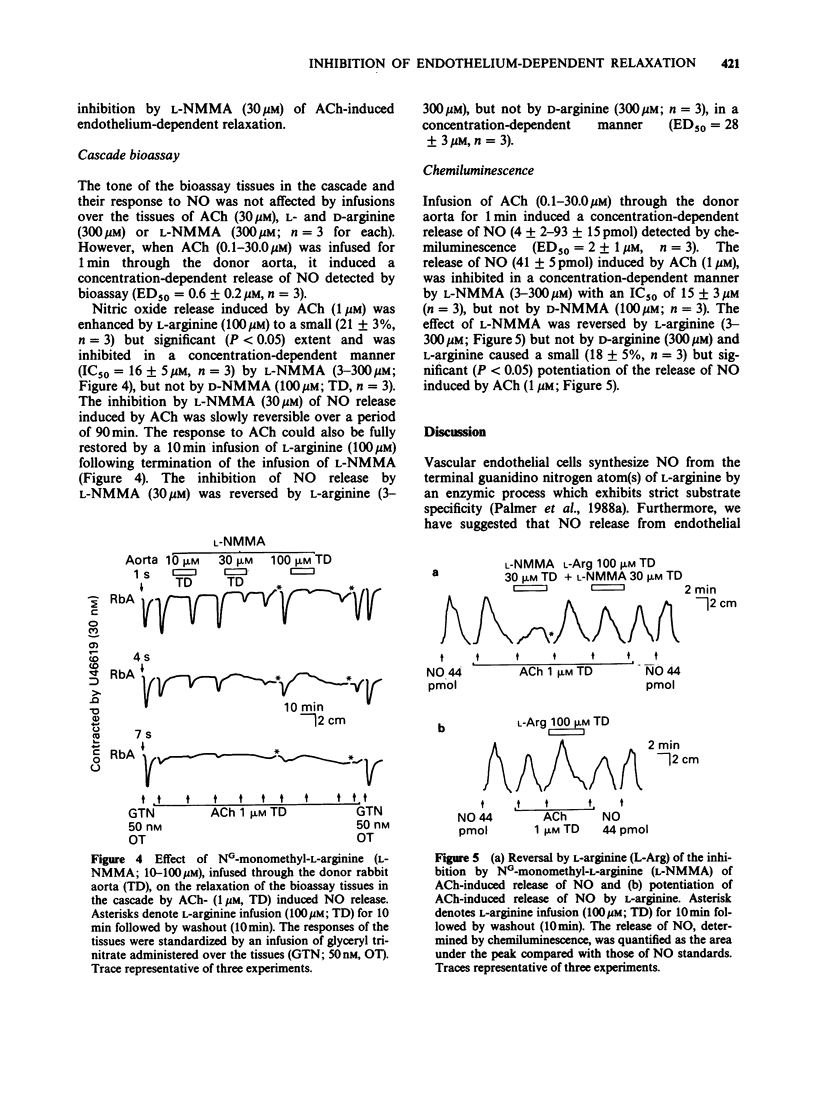
These references are in PubMed. This may not be the complete list of references from this article.
- Amber I. J., Hibbs J. B., Jr, Taintor R. R., Vavrin Z. The L-arginine dependent effector mechanism is induced in murine adenocarcinoma cells by culture supernatant from cytotoxic activated macrophages. J Leukoc Biol. 1988 Feb;43(2):187–192. doi: 10.1002/jlb.43.2.187. [DOI] [PubMed] [Google Scholar]
- Amezcua J. L., Dusting G. J., Palmer R. M., Moncada S. Acetylcholine induces vasodilatation in the rabbit isolated heart through the release of nitric oxide, the endogenous nitrovasodilator. Br J Pharmacol. 1988 Nov;95(3):830–834. doi: 10.1111/j.1476-5381.1988.tb11711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feletou M., Vanhoutte P. M. Endothelium-dependent hyperpolarization of canine coronary smooth muscle. Br J Pharmacol. 1988 Mar;93(3):515–524. doi: 10.1111/j.1476-5381.1988.tb10306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Granger D. L., Hibbs J. B., Jr, Perfect J. R., Durack D. T. Specific amino acid (L-arginine) requirement for the microbiostatic activity of murine macrophages. J Clin Invest. 1988 Apr;81(4):1129–1136. doi: 10.1172/JCI113427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryglewski R. J., Moncada S., Palmer R. M. Bioassay of prostacyclin and endothelium-derived relaxing factor (EDRF) from porcine aortic endothelial cells. Br J Pharmacol. 1986 Apr;87(4):685–694. doi: 10.1111/j.1476-5381.1986.tb14586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryglewski R. J., Palmer R. M., Moncada S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature. 1986 Apr 3;320(6061):454–456. doi: 10.1038/320454a0. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Taintor R. R., Vavrin Z. Macrophage cytotoxicity: role for L-arginine deiminase and imino nitrogen oxidation to nitrite. Science. 1987 Jan 23;235(4787):473–476. doi: 10.1126/science.2432665. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Vavrin Z., Taintor R. R. L-arginine is required for expression of the activated macrophage effector mechanism causing selective metabolic inhibition in target cells. J Immunol. 1987 Jan 15;138(2):550–565. [PubMed] [Google Scholar]
- Iyengar R., Stuehr D. J., Marletta M. A. Macrophage synthesis of nitrite, nitrate, and N-nitrosamines: precursors and role of the respiratory burst. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6369–6373. doi: 10.1073/pnas.84.18.6369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin W., Villani G. M., Jothianandan D., Furchgott R. F. Selective blockade of endothelium-dependent and glyceryl trinitrate-induced relaxation by hemoglobin and by methylene blue in the rabbit aorta. J Pharmacol Exp Ther. 1985 Mar;232(3):708–716. [PubMed] [Google Scholar]
- Moncada S., Radomski M. W., Palmer R. M. Endothelium-derived relaxing factor. Identification as nitric oxide and role in the control of vascular tone and platelet function. Biochem Pharmacol. 1988 Jul 1;37(13):2495–2501. doi: 10.1016/0006-2952(88)90236-5. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ashton D. S., Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988 Jun 16;333(6174):664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Rees D. D., Ashton D. S., Moncada S. L-arginine is the physiological precursor for the formation of nitric oxide in endothelium-dependent relaxation. Biochem Biophys Res Commun. 1988 Jun 30;153(3):1251–1256. doi: 10.1016/s0006-291x(88)81362-7. [DOI] [PubMed] [Google Scholar]
- Patthy A., Bajusz S., Patthy L. Preparation and characterization of Ng-mono-, di- and trimethylated arginines. Acta Biochim Biophys Acad Sci Hung. 1977;12(3):191–196. [PubMed] [Google Scholar]
- Radomski M. W., Palmer R. M., Moncada S. The anti-aggregating properties of vascular endothelium: interactions between prostacyclin and nitric oxide. Br J Pharmacol. 1987 Nov;92(3):639–646. doi: 10.1111/j.1476-5381.1987.tb11367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radomski M. W., Palmer R. M., Moncada S. The role of nitric oxide and cGMP in platelet adhesion to vascular endothelium. Biochem Biophys Res Commun. 1987 Nov 13;148(3):1482–1489. doi: 10.1016/s0006-291x(87)80299-1. [DOI] [PubMed] [Google Scholar]
- Rees D. D., Palmer R. M., Moncada S. Effect of SKF 525A on the release of nitric oxide and prostacyclin from endothelial cells. Eur J Pharmacol. 1988 May 20;150(1-2):149–154. doi: 10.1016/0014-2999(88)90761-3. [DOI] [PubMed] [Google Scholar]
- Rimele T. J., Sturm R. J., Adams L. M., Henry D. E., Heaslip R. J., Weichman B. M., Grimes D. Interaction of neutrophils with vascular smooth muscle: identification of a neutrophil-derived relaxing factor. J Pharmacol Exp Ther. 1988 Apr;245(1):102–111. [PubMed] [Google Scholar]
- Schmidt H. H., Klein M. M., Niroomand F., Böhme E. Is arginine a physiological precursor of endothelium-derived nitric oxide? Eur J Pharmacol. 1988 Mar 29;148(2):293–295. doi: 10.1016/0014-2999(88)90578-x. [DOI] [PubMed] [Google Scholar]
- Schneider E., Kamoun P. P., Migliore-Samour D., Dy M. A new enzymatic pathway of citrullinogenesis in murine hemopoietic cells. Biochem Biophys Res Commun. 1987 Apr 29;144(2):829–835. doi: 10.1016/s0006-291x(87)80039-6. [DOI] [PubMed] [Google Scholar]


