Abstract
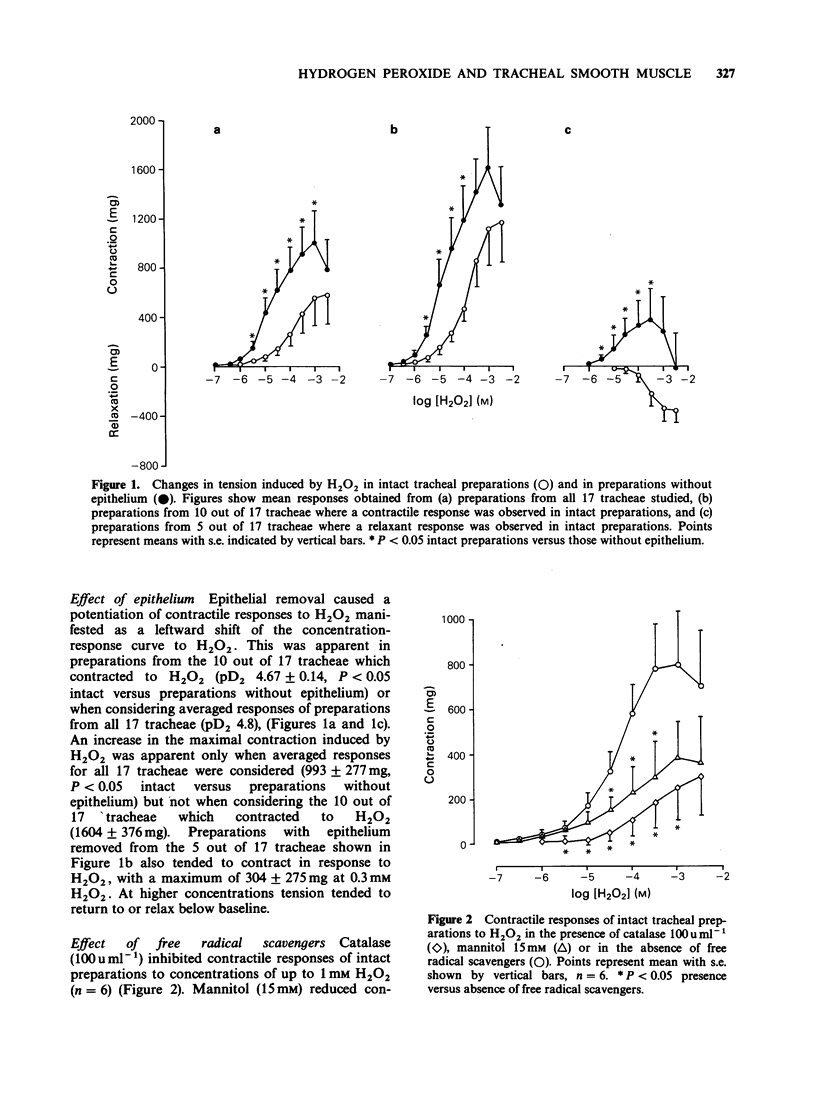
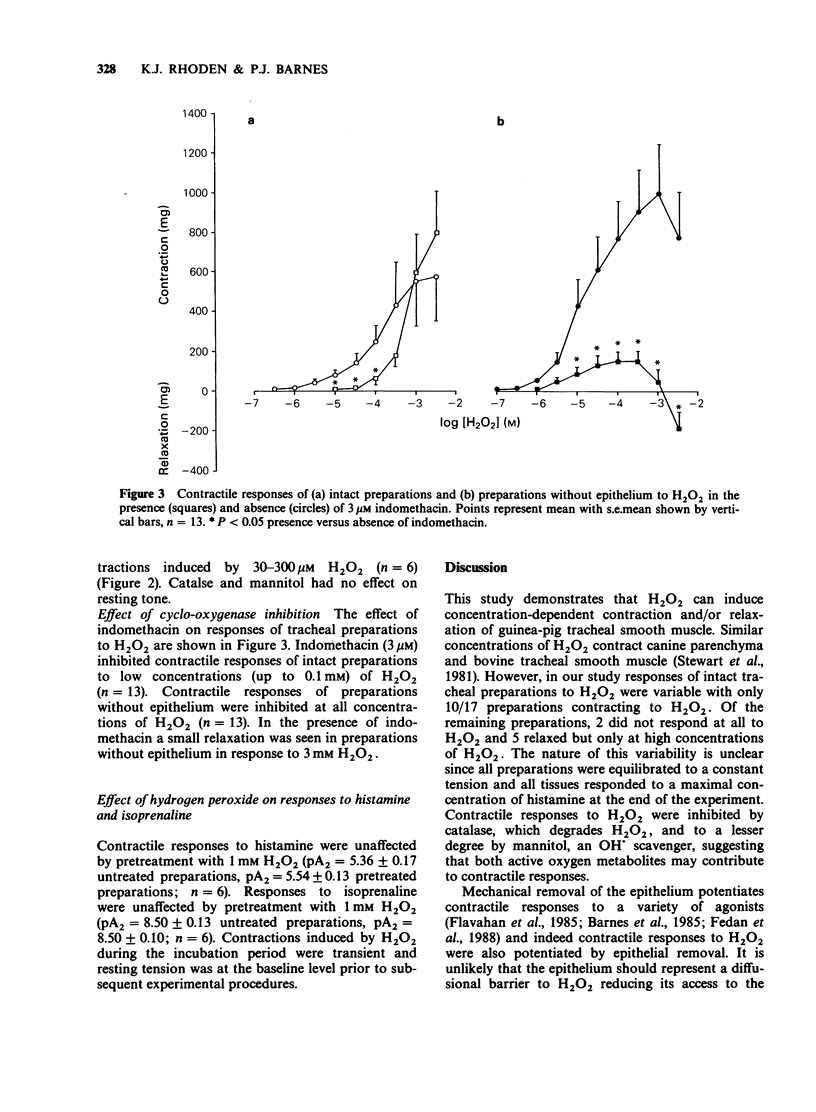
1. Hydrogen peroxide (H2O2) (0.1 microM-3 mM) induced variable contractions of guinea-pig isolated trachea which were attenuated by catalase (100 u ml-1) and mannitol (15 mM) suggesting that contractions were induced by H2O2 and/or the hydroxyl anion. 2. Epithelial removal potentiated contractile responses of tracheal preparations to H2O2 with a leftward shift of the concentration-response curve and an increase in the maximal response. 3. Indomethacin (3 microM) inhibited contractions to H2O2 of intact preparations and preparations without epithelium suggesting that contractions may be mediated by cyclo-oxygenase products. Intact preparations (but not preparations without epithelium) contracted in response to high concentrations (greater than 0.1 mM) of H2O2 in the presence of indomethacin suggesting that other excitatory factor(s) released by the epithelium may induce contraction. 4. Preincubation of intact tracheal preparations with H2O2 (1 mM) for 1 h had no effect on responses to histamine or isoprenaline. 5. These results suggest that hydrogen peroxide generated during the inflammatory process may play a role in bronchoconstriction.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babior B. M., Kipnes R. S., Curnutte J. T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973 Mar;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes P. J., Cuss F. M., Palmer J. B. The effect of airway epithelium on smooth muscle contractility in bovine trachea. Br J Pharmacol. 1985 Nov;86(3):685–691. doi: 10.1111/j.1476-5381.1985.tb08946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigham K. L. Role of free radicals in lung injury. Chest. 1986 Jun;89(6):859–863. doi: 10.1378/chest.89.6.859. [DOI] [PubMed] [Google Scholar]
- Burman W. J., Martin W. J., 2nd Oxidant-mediated ciliary dysfunction. Possible role in airway disease. Chest. 1986 Mar;89(3):410–413. doi: 10.1378/chest.89.3.410. [DOI] [PubMed] [Google Scholar]
- DeChatelet L. R., Shirley P. S., McPhail L. C., Huntley C. C., Muss H. B., Bass D. A. Oxidative metabolism of the human eosinophil. Blood. 1977 Sep;50(3):525–535. [PubMed] [Google Scholar]
- Drath D. B., Karnovsky M. L. Superoxide production by phagocytic leukocytes. J Exp Med. 1975 Jan 1;141(1):257–262. doi: 10.1084/jem.141.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engels F., Oosting R. S., Nijkamp F. P. Pulmonary macrophages induce deterioration of guinea-pig tracheal beta-adrenergic function through release of oxygen radicals. Eur J Pharmacol. 1985 Apr 23;111(1):143–144. doi: 10.1016/0014-2999(85)90127-x. [DOI] [PubMed] [Google Scholar]
- Flavahan N. A., Aarhus L. L., Rimele T. J., Vanhoutte P. M. Respiratory epithelium inhibits bronchial smooth muscle tone. J Appl Physiol (1985) 1985 Mar;58(3):834–838. doi: 10.1152/jappl.1985.58.3.834. [DOI] [PubMed] [Google Scholar]
- Hay D. W., Muccitelli R. M., Horstemeyer D. L., Wilson K. A., Raeburn D. Demonstration of the release of an epithelium-derived inhibitory factor from a novel preparation of guinea-pig trachea. Eur J Pharmacol. 1987 Apr 14;136(2):247–250. doi: 10.1016/0014-2999(87)90719-9. [DOI] [PubMed] [Google Scholar]
- Kramer K., Rademaker B., Rozendal W. H., Timmerman H., Bast A. Influence of lipid peroxidation on beta-adrenoceptors. FEBS Lett. 1986 Mar 17;198(1):80–84. doi: 10.1016/0014-5793(86)81188-7. [DOI] [PubMed] [Google Scholar]
- Nishida Y., Suzuki S., Miyamoto T. Biphasic contraction of isolated guinea pig tracheal chains by superoxide radical. Inflammation. 1985 Sep;9(3):333–337. doi: 10.1007/BF00916281. [DOI] [PubMed] [Google Scholar]
- Sacks T., Moldow C. F., Craddock P. R., Bowers T. K., Jacob H. S. Oxygen radicals mediate endothelial cell damage by complement-stimulated granulocytes. An in vitro model of immune vascular damage. J Clin Invest. 1978 May;61(5):1161–1167. doi: 10.1172/JCI109031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart R. M., Weir E. K., Montgomery M. R., Niewoehner D. E. Hydrogen peroxide contracts airway smooth muscle: a possible endogenous mechanism. Respir Physiol. 1981 Sep;45(3):333–342. doi: 10.1016/0034-5687(81)90016-5. [DOI] [PubMed] [Google Scholar]
- Tate R. M., Morris H. G., Schroeder W. R., Repine J. E. Oxygen metabolites stimulate thromboxane production and vasoconstriction in isolated saline-perfused rabbit lungs. J Clin Invest. 1984 Aug;74(2):608–613. doi: 10.1172/JCI111458. [DOI] [PMC free article] [PubMed] [Google Scholar]


