Abstract
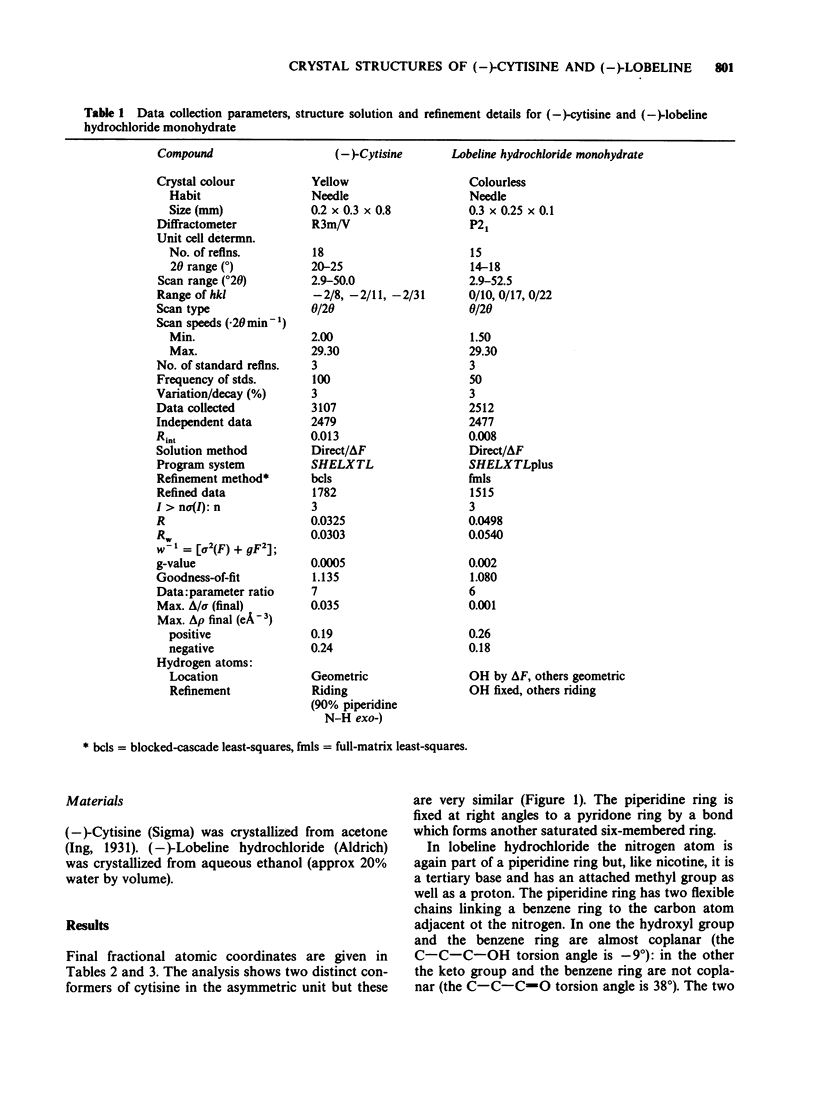
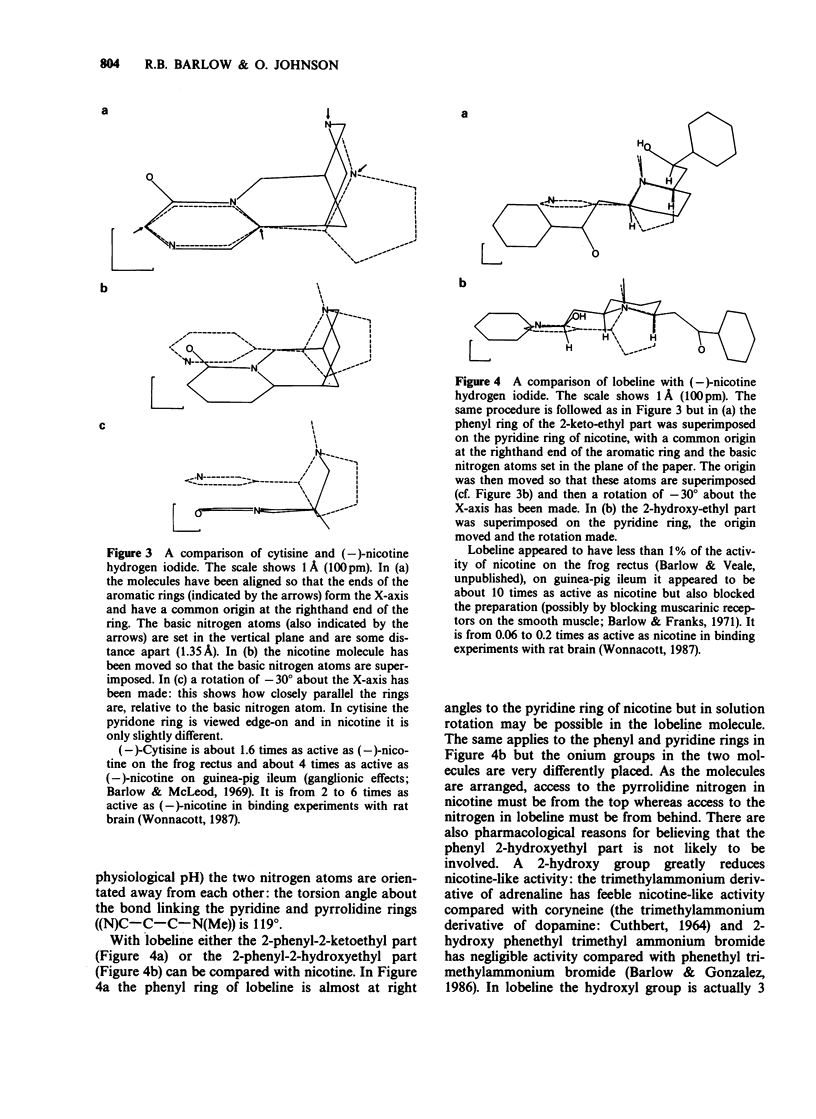
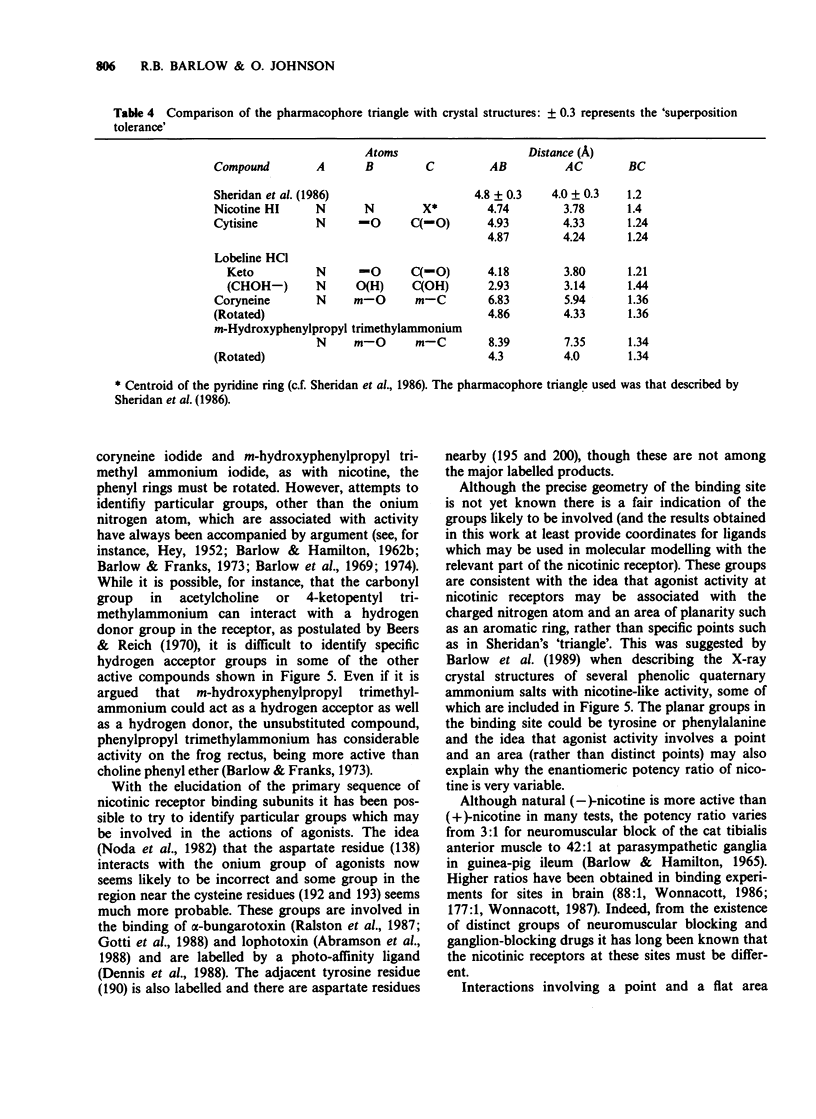
1. Although (-)-cytisine is a rigid structure, it occurs in the crystal in two distinct but very similar conformations in which the pyridone ring is tilted relative to the charged nitrogen atom at much the same angle as the pyridine ring is in (-)-nicotine hydrogen iodide. The carbonyl group in the pyridone ring of (-)-cytisine, however, is on the side of the ring opposite to pyridine nitrogen in (-)-nicotine. 2. The pKa of (-)-lobeline HCl at 25 degrees C is 8.6 (approx), indicating that (-)-lobeline is at least 90% in the protonated form at physiological pH (7.6). It is probably the phenyl 2-keto-ethyl part of (-)-lobeline, rather than the phenyl 2-hydroxy-ethyl part, which interacts with the receptor. 3. The combination within one molecule of a charged ('onium') nitrogen atom lying out of the plane of, and some distance (4.5-6.5 A) from, an aromatic ring is common to many compounds with nicotine-like activity (e.g. nicotine, cytisine, choline phenyl ether bromide, dimethyl-phenyl-piperazinium (DMPP) iodide, coryneine iodide and m-hydroxyphenylpropyl trimethyl ammonium iodide). In some molecules the aromatic ring can be replaced by an unsaturated group, such as carbonyl (e.g. acetylcholine) or double-bonds (e.g. anatoxin). 4. Activity at nicotinic receptors appears to involve interactions between the positively charged nitrogen atom and a negatively charged group, probably close to cysteine residues 192 and 193 in the receptor. It is suggested that rather than specific groups in the molecule also being involved, activity at nicotinic receptors depends on interactions between a flat part of the drug containing double-bonds, or systems of double bonds, and a planar area in the receptor, possibly tyrosine or phenylalanine residues.
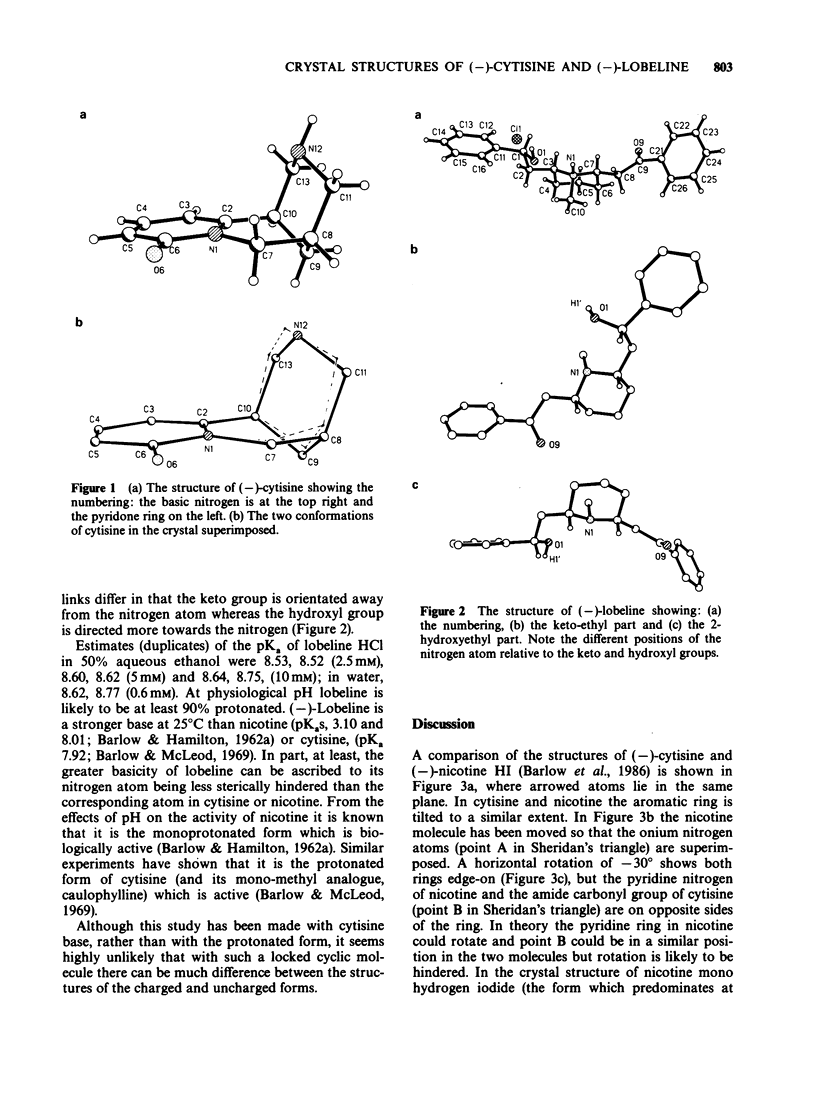
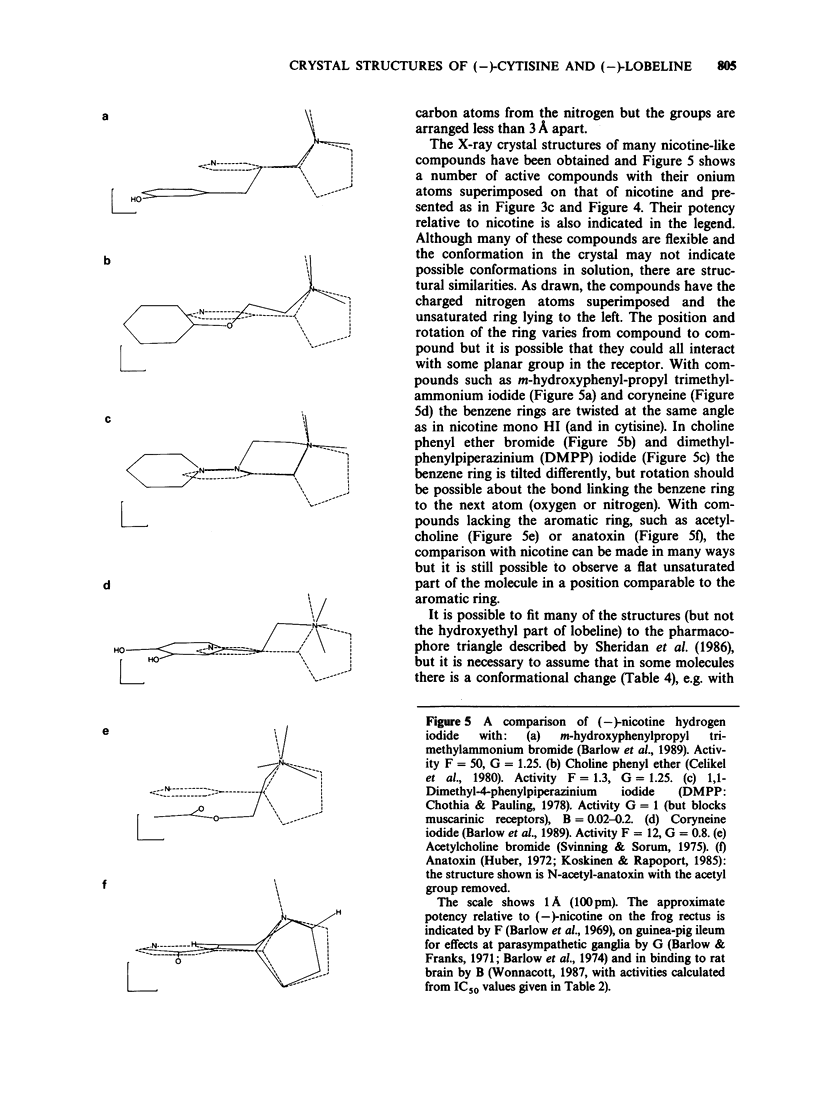
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramson S. N., Culver P., Kline T., Li Y., Guest P., Gutman L., Taylor P. Lophotoxin and related coral toxins covalently label the alpha-subunit of the nicotinic acetylcholine receptor. J Biol Chem. 1988 Dec 5;263(34):18568–18573. [PubMed] [Google Scholar]
- Armstrong J., Barlow R. B. The ionization of phenolic amines, including apomorphine, dopamine and catecholamines and an assessment of zwitterion constants. Br J Pharmacol. 1976 Aug;57(4):501–516. doi: 10.1111/j.1476-5381.1976.tb10377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow R. B., Bowman F., Ison R. R., McQueen D. S. The specificity of some agonists and antagonists for nicotine-sensitive receptors in ganglia. Br J Pharmacol. 1974 Aug;51(4):585–597. doi: 10.1111/j.1476-5381.1974.tb09678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow R. B., Franks F. M. A comparison of phenylalkyl- and phenoxyalkyl- trimethylammonium and triethylammonium salts; their apparent molal volumes at infinite dilution and effects on the frog rectus and guinea-pig ileum preparations. Br J Pharmacol. 1973 Nov;49(3):480–489. doi: 10.1111/j.1476-5381.1973.tb17258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow R. B., Franks F. Specificity of some ganglion stimulants. Br J Pharmacol. 1971 May;42(1):137–142. doi: 10.1111/j.1476-5381.1971.tb07093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow R. B., González J. L. Effects of hydroxyl groups on nicotine-like activity and on size in solution. Arch Farmacol Toxicol. 1986 Aug-Dec;12(2-3):87–98. [PubMed] [Google Scholar]
- Barlow R. B., Johnson O., Howard J. A., Walton D. C., Koellner G. A comparison of the crystal structures of some quaternary trimethylammonium salts related to dopamine and noradrenaline with those of the corresponding amines: a comment on their nicotine-like biological activities. Acta Crystallogr B. 1989 Aug 1;45(Pt 4):396–404. doi: 10.1107/s0108768189002740. [DOI] [PubMed] [Google Scholar]
- Barlow R. B., McLeod L. J. Some studies on cytisine and its methylated derivatives. Br J Pharmacol. 1969 Jan;35(1):161–174. doi: 10.1111/j.1476-5381.1969.tb07977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow R. B., Thompson G. M., Scott N. C. The affinity and activity of compounds related to nicotine on the rectus abdominis muscle of the frog (Rana pipiens). Br J Pharmacol. 1969 Nov;37(3):555–584. doi: 10.1111/j.1476-5381.1969.tb08496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beers W. H., Reich E. Structure and activity of acetylcholine. Nature. 1970 Dec 5;228(5275):917–922. doi: 10.1038/228917a0. [DOI] [PubMed] [Google Scholar]
- CUTHBERT M. F. RELATIVE ACTIONS OF QUATERNARY METHYL DERIVATIVES OF TYRAMINE, DOPAMINE AND NORADRENALINE. Br J Pharmacol Chemother. 1964 Aug;23:55–65. doi: 10.1111/j.1476-5381.1964.tb01566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changeux J. P., Devillers-Thiéry A., Chemouilli P. Acetylcholine receptor: an allosteric protein. Science. 1984 Sep 21;225(4668):1335–1345. doi: 10.1126/science.6382611. [DOI] [PubMed] [Google Scholar]
- Criado M., Sarin V., Fox J. L., Lindstrom J. Evidence that the acetylcholine binding site is not formed by the sequence alpha 127-143 of the acetylcholine receptor. Biochemistry. 1986 May 20;25(10):2839–2846. doi: 10.1021/bi00358a015. [DOI] [PubMed] [Google Scholar]
- Dennis M., Giraudat J., Kotzyba-Hibert F., Goeldner M., Hirth C., Chang J. Y., Lazure C., Chrétien M., Changeux J. P. Amino acids of the Torpedo marmorata acetylcholine receptor alpha subunit labeled by a photoaffinity ligand for the acetylcholine binding site. Biochemistry. 1988 Apr 5;27(7):2346–2357. doi: 10.1021/bi00407a016. [DOI] [PubMed] [Google Scholar]
- Gotti C., Frigerio F., Bolognesi M., Longhi R., Racchetti G., Clementi F. Nicotinic acetylcholine receptor: a structural model for alpha-subunit peptide 188-201, the putative binding site for cholinergic agents. FEBS Lett. 1988 Feb 8;228(1):118–122. doi: 10.1016/0014-5793(88)80598-2. [DOI] [PubMed] [Google Scholar]
- HEY P. On relationships between structure and nicotine-like stimulant activity in choline esters and ethers. Br J Pharmacol Chemother. 1952 Mar;7(1):117–129. doi: 10.1111/j.1476-5381.1952.tb00697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao P. N., Karlin A. Acetylcholine receptor binding site contains a disulfide cross-link between adjacent half-cystinyl residues. J Biol Chem. 1986 Jun 25;261(18):8085–8088. [PubMed] [Google Scholar]
- Koskinen A. M., Rapoport H. Synthetic and conformational studies on anatoxin-a: a potent acetylcholine agonist. J Med Chem. 1985 Sep;28(9):1301–1309. doi: 10.1021/jm00147a032. [DOI] [PubMed] [Google Scholar]
- McCormick D. J., Atassi M. Z. Localization and synthesis of the acetylcholine-binding site in the alpha-chain of the Torpedo californica acetylcholine receptor. Biochem J. 1984 Dec 15;224(3):995–1000. doi: 10.1042/bj2240995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulac-Jericevic B., Atassi M. Z. Segment alpha 182-198 of Torpedo californica acetylcholine receptor contains second toxin-binding region and binds anti-receptor antibodies. FEBS Lett. 1986 Apr 7;199(1):68–74. doi: 10.1016/0014-5793(86)81225-x. [DOI] [PubMed] [Google Scholar]
- Noda M., Takahashi H., Tanabe T., Toyosato M., Furutani Y., Hirose T., Asai M., Inayama S., Miyata T., Numa S. Primary structure of alpha-subunit precursor of Torpedo californica acetylcholine receptor deduced from cDNA sequence. Nature. 1982 Oct 28;299(5886):793–797. doi: 10.1038/299793a0. [DOI] [PubMed] [Google Scholar]
- Oblas B., Singer R. H., Boyd N. D. Location of a polypeptide sequence within the alpha-subunit of the acetylcholine receptor containing the cholinergic binding site. Mol Pharmacol. 1986 Jun;29(6):649–656. [PubMed] [Google Scholar]
- Ralston S., Sarin V., Thanh H. L., Rivier J., Fox J. L., Lindstrom J. Synthetic peptides used to locate the alpha-bungarotoxin binding site and immunogenic regions on alpha subunits of the nicotinic acetylcholine receptor. Biochemistry. 1987 Jun 16;26(12):3261–3266. doi: 10.1021/bi00386a004. [DOI] [PubMed] [Google Scholar]
- Sheridan R. P., Nilakantan R., Dixon J. S., Venkataraghavan R. The ensemble approach to distance geometry: application to the nicotinic pharmacophore. J Med Chem. 1986 Jun;29(6):899–906. doi: 10.1021/jm00156a005. [DOI] [PubMed] [Google Scholar]
- Spivak C. E., Gund T. M., Liang R. F., Waters J. A. Structural and electronic requirements for potent agonists at a nicotinic receptor. Eur J Pharmacol. 1986 Jan 14;120(1):127–131. doi: 10.1016/0014-2999(86)90652-7. [DOI] [PubMed] [Google Scholar]
- Waters J. A., Spivak C. E., Hermsmeier M., Yadav J. S., Liang R. F., Gund T. M. Synthesis, pharmacology, and molecular modeling studies of semirigid, nicotinic agonists. J Med Chem. 1988 Mar;31(3):545–554. doi: 10.1021/jm00398a010. [DOI] [PubMed] [Google Scholar]
- Watters D., Maelicke A. Organization of ligand binding sites at the acetylcholine receptor: a study with monoclonal antibodies. Biochemistry. 1983 Apr 12;22(8):1811–1819. doi: 10.1021/bi00277a011. [DOI] [PubMed] [Google Scholar]
- Wonnacott S. Brain nicotine binding sites. Hum Toxicol. 1987 Sep;6(5):343–353. doi: 10.1177/096032718700600502. [DOI] [PubMed] [Google Scholar]
- Wonnacott S. alpha-Bungarotoxin binds to low-affinity nicotine binding sites in rat brain. J Neurochem. 1986 Dec;47(6):1706–1712. doi: 10.1111/j.1471-4159.1986.tb13078.x. [DOI] [PubMed] [Google Scholar]


