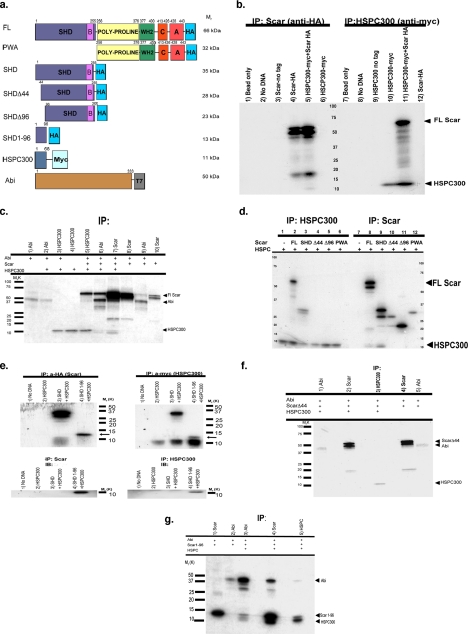
Figure 2.
The N-terminus of Scar binds HSPC300 and Abi in vitro. (a) Constructs used in these experiments. Numbers represent position of amino acids relative to the N-terminus. Relative molecular weights are indicated on the right. All Scar constructs contain C-terminal HA tag. FL, full-length Scar; SHD, construct containing the Scar homology domain (SHD) and Basic (B) regions; PWA, construct containing the poly-proline region, WH2, connecting (C), and acidic (A) regions; SHDΔ44, SHD construct lacking the first 44 amino acids; SHDΔ96, SHD construct lacking the first 96 amino acids. SHD1-96, Scar construct containing only amino acids 1–96; HSPC300, HSPC300 protein with C-terminal myc tag; Abi protein, amino acids 1–333 with C-terminal T7 peptide tag. (b) SDS-PAGE of in vitro binding and coimmunoprecipitation assays. Scar constructs with a C-terminal HA tag and HSPC300 with C-terminal myc tag were separately transcribed and translated in vitro, mixed, and then immunoprecipitated with protein G agarose beads conjugated to anti-HA (lanes 1–6) or anti-myc antibodies (lanes 7–12). Bead only controls representing lysates mixed with agarose beads are shown in lanes 1 and 7. Immunoprecipitates of lysate containing no added DNA are shown in lanes 2 and 8. Lysates containing PCR-generated DNAs of untagged Scar (lane 3) or untagged HSPC300 (lane 9) immunoprecipitated with anti-HA or anti-myc–conjugated beads, respectively, are shown. Transcription and translation of untagged proteins was verified (data not shown). Lysates containing both Scar-HA and HSPC300-myc immunoprecipitated with anti-HA (lane 5) or anti-myc (lane 11) are shown. FL Scar-HA migrates at ∼66 kDa; HSPC300 migrates at ∼11 kDa. Controls for anti-HA bead specificity are shown in lanes 4 and 6. Controls for anti-myc bead specificity are shown in lanes 10 and 12. (c) Scar-HA, Abi-T7, and HSPC300-myc were cotranslated and immunoprecipitated in various combinations. Lysates immunoprecipitated with anti-T7 (Abi), anti-HA (Scar), or anti-myc–conjugated agarose beads are shown. Assays were done as described in b. Molecular-weight marker sizes are indicated on the left. (d) Amino acids 1–44 of Scar are necessary to bind HSPC300 in vitro. Assay was done as described in b. Proteins added in binding assay are indicated for each lane. Lysates immunoprecipitated for HSPC300 using anti-myc–conjugated agarose beads (lanes 1–6) or for Scar using anti-HA–conjugated agarose beads (lanes 7–12) are shown. (e) Amino acids 1–96 of Scar are sufficient to bind HSPC300 in vitro. Assays were as in b. Lysates were immunoprecipitated for Scar using anti-HA bound agarose beads. The gels were either detected for 35S-Met (top panels) or immunoblotted with anti-HA antibody (bottom panels). Control lysate containing no added DNA is shown in lane 1. HSCP300 control is shown in lane 2. Lane 3 represents lysate containing both SHD-HA and HSPC300-myc proteins. Lane 4 represents lysate containing 1–96 fragment of SHD domain and HSPC300. (f) Amino acids 1–44 of Scar are necessary to bind to Abi. ScarΔ44-HA (full-length Scar minus the first 44 amino acids), Abi-T7, and HSPC300-myc were cotranslated and immunoprecipitated with anti-T7– conjugated agarose beads (lane 1), anti-HA–conjugated agarose beads (lane 2), or anti-myc–conjugated agarose beads (lane 3). ScarΔ44-HA (full-length Scar minus the first 44 amino acids) and Abi-T7 were cotranslated and immunoprecipitated with anti-HA–conjugated agarose beads (lane 4) or anti-T–conjugated agarose beads (lane 5). (g) Amino acids 1–96 of Scar are sufficient to bind Abi in the presence of HSPC300. Scar SHD1-96-HA and Abi-T7 were cotranslated and immunoprecipitated with anti-HA–conjugated agarose beads (lane 1) or anti-T7–conjugated agarose beads (lane 2). Scar SHD1-96-HA, Abi-T7, and HSPC300-myc were cotranslated and immunoprecipitated with anti-T7–conjugated agarose beads (lane 3), anti-HA–conjugated agarose beads (lane 4), or anti-myc–conjugated agarose beads (lane 5).