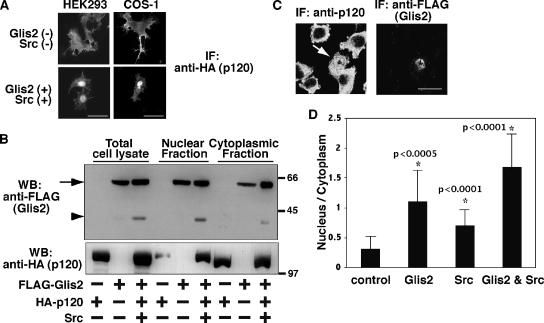
Figure 4.
Nuclear translocation of p120 by Glis2 and Src. (A) Immunofluorescence staining of exogenous p120. HA-p120 was transiently transfected in HEK293 and COS-1 cells with or without coexpression of FLAG-Glis2 and Src. The localization of p120 was examined by epifluorescent microscopy by using anti-HA antibody. Bars, 20 μm. (B) Both Glis2 and Glis2 ΔC localize in the nucleus. FLAG-Glis2 was coexpressed with HA-p120 and Src, and nuclear and cytoplasmic fractions were isolated and examined by Western blotting using anti-FLAG and anti-HA antibodies. The arrow and arrowhead indicate the positions of full-length Glis2 and its cleavage product Glis2 ΔC, respectively. (C) Immunofluorescence staining of endogenous p120. FLAG-Glis2 was transiently transfected in L fibroblast cells. The localization of endogenous p120 and exogenous Glis2 was examined by confocal microscopy by using anti-p120 and anti-FLAG antibodies, respectively. The arrow indicates the Glis2-positive cell, which contains nuclear p120. Bar, 20 μm. (D) Quantification of the nuclear translocation of endogenous p120. FLAG-Glis2 and/or Src were transiently transfected in L fibroblast cells. The empty pEGFP vector was cotransfected when FLAG-Glis2 was not transfected to identify transfected cells. The location of the nucleus was confirmed by Hoechst staining. Immunofluorescent staining intensity with anti-p120 antibody was measured in a defined region (diameter 1.5 μm) in the nucleus or cytosol, and the ratio of nucleus/cytoplasm staining intensity was analyzed in 15 cells per transfection. Asterisk (*) indicates significantly higher than control cells (p value is shown beside the asterisk).