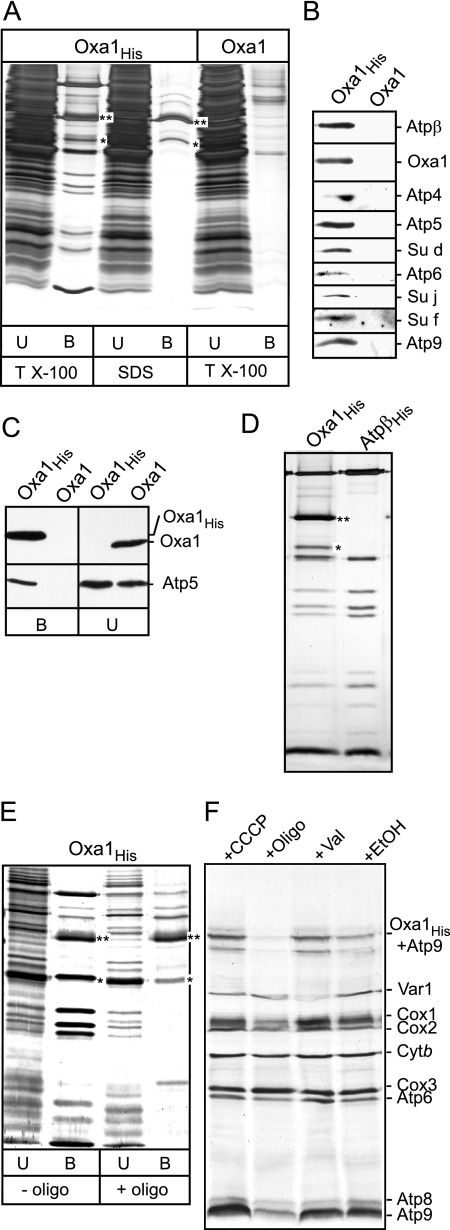
Figure 5.
The ATP synthase complex can be copurified together with Oxa1. (A) Mitochondria isolated from strains expressing Oxa1His or Oxa1 were solubilized with buffer containing either 1% Triton X-100 (TX-100) or 0.1% SDS as indicated and incubated with Ni-NTA beads as described in Materials and Methods. Twenty percent of the unbound material (U) and the Ni-NTA beads purified material (indicated by B) were analyzed by SDS-PAGE and silver-staining. (B) Mitochondria isolated from strains expressing Oxa1His or Oxa1 (GAL-Oxa1 and GAL-Oxa1His, respectively) were solubilized with buffer containing 1% Triton X-100, as indicated, and were incubated with Ni-NTA beads. The Ni-NTA–purified material was analyzed by SDS-PAGE and Western blotting with antisera against ATP synthase subunits Atpβ, Atp4, Atp5, Atp6, Su d, Su j, Su f, Atp9, and Oxa1 as indicated. (C) Mitochondria harboring expressed Oxa1 or Oxa1His proteins were lysed in Triton X-100 containing buffer and were subjected to Ni-NTA purification. The purified material (indicated by B) and 10% of the unbound material (U) were subjected to SDS-PAGE, Western blotting, and immunedecoration with Oxa1 and Atp5 antisera as indicated. (D) Mitochondria harboring expressed Oxa1His (200 μg protein) or histidine-tagged Atpβ (AtpβHis; 50 μg protein) were solubilized with buffer containing 1% Triton X-100 as indicated and incubated with Ni-NTA beads. The Ni-NTA–purified material was analyzed by SDS-PAGE and silver staining. (E) Mitochondria harboring Oxa1His were solubilized with buffer containing 1% Triton X-100 in the absence or presence of oligomycin (10 μg/ml) as indicated. Samples were further analyzed as described in A. (F) In organello translation was performed in Oxa1His containing mitochondria in the presence of CCCP (100 μM), oligomycin (10 μg/ml +oligo), valinomycin (0.5 μM, +Val), or ethanol (+EtOH) as a control, for 30 min at 25°C. After the addition of cold methionine, mitochondria were reisolated and subjected to SDS-PAGE and autoradiography. The position of the radiolabeled Atp9-Oxa1His SDS-resistant complex is indicated. The asterisks (** and *) in A, C, and D indicate the positions of Oxa1His and a C-terminal degradation product of Oxa1His, respectively.