Figure 1.
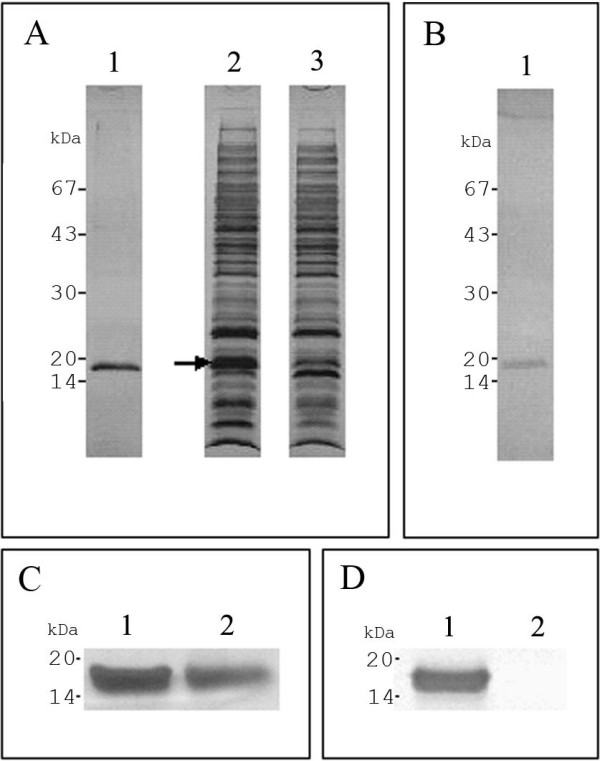
S. enterica has an 18-kDa jacalin binding protein. SDS-PAGE analysis of the purified antigen of S. enterica stained with silver nitrate (A and C) and PAS/silver nitrate (B and D). An overnight culture of S. enterica Typhimurium UK-1 was pelleted, washed, and sonicated. After centrifugation, the supernatant was collected for chromatography on a jacalin column immobilized on Sepharose®. A single band of approximately 18 kDa delayed in the column was eluted with D-galactose and stained by both methods (lane 1, A and B). An equivalent band can be visualized (arrow) in the crude extract before chromatography (lane 2, A), and it is much less intense in the non-delayed fraction (lane 3, A). Dps was also submitted to beta-elimination reaction with NH4OH (C and D). Intact (native) J-Dps was stained by both methods (lane 1, C and D), whereas the NH4OH-treated protein was stained with silver nitrate but not with PAS (lane 2, C and D, respectively). The 18-kDa protein displayed an N-terminal sequence similar to that of the DNA-binding protein (Dps) of S. enterica Typhimurium LT-2. Molecular mass markers: lactoalbumin (14 kDa), trypsin inhibitor (20 kDa), carbonic anhydrase (30 kDa), hen egg albumin (43 kDa), and bovine serum albumin (67 kDa).
