Figure 4. RIIα D/D domain forms an asymmetric pocket upon D-AKAP2 binding.
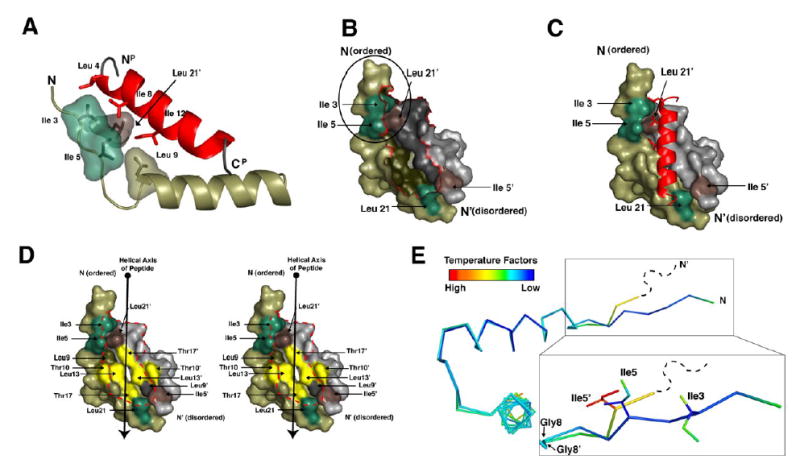
(A) Ile3R, Ile5R, Leu9R and Leu21R’ make critical contacts with peptide residues Leu4P, Ala5P, Ile8P, and Ile12P. These residues are highlighted to show their proximity to each other and the importance of the branching of the isoleucine side chains.
(B and C) The surface of RIIα D/D domain is displayed alone and with the AKAP. The Ile3R, Ile5R, Leu21R are shown in green. Ile5R’ and Leu21R’ are shown in brown. These residues are highlighted to illustrate the two potential docking sites created Ile3R, Ile5R and Leu21R’ and Ile5R’ and Leu21R. The N-terminus of RIIαD/D, including Ile3R and Ile5R (circled in B) is ordered in the structure and cluster with Leu21R’ to form a hydrophobic site that interacts with a number of side chains from the peptide. However, the N-terminal extension of RIIαD/D’ is disordered in our crystal, so it cannot be seen in our model.
(D) The hydrophobic groove of the RIIα D/D domain is illustrated with a stereo-representation of the D/D domain surface. Residues that line this groove and contribute to AKAP docking are highlighted. Residues colored in yellow represent the center of the groove, which are important for binding specificity.
(E) The two protomers from the D/D domain are aligned and colored according to B-factor to show the asymmetry more precisely. The RIIαD/D’ protomer is disordered at the N-terminus beyond residue 4 and residue 5 is relatively dynamic according to B-factors. However, this region is well ordered on the protomer that contacts the N-terminus of the peptide. The position of Ile5R and Ile5R’ is different due to changes around Gly8R and Gly8R’.
