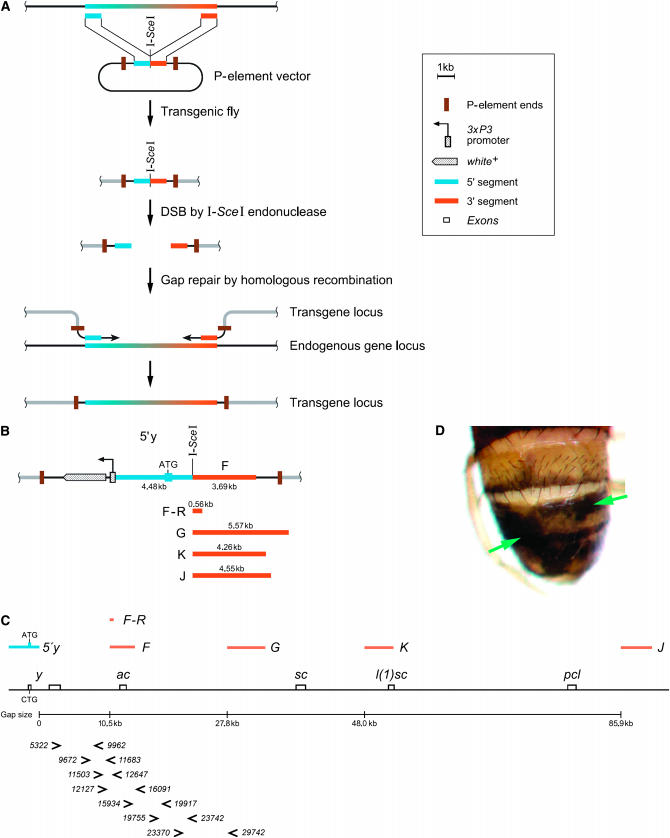
Figure 1.—
(A) Principle of the construction of transgenes in vivo. To copy a desired region from the endogenous locus into a transgene, two segments (blue and orange boxes) at both ends of the target DNA are cloned into an insect transformation vector. An I-SceI recognition site connects the two segments. This element is integrated into the Drosophila genome at a position different from the locus of interest. By crossing with flies expressing the endonuclease I-SceI, a DSB is introduced in the transgene between the two segments. Subsequently, the transgene acquires the missing region from the endogenous locus by gap repair. (B–D) In vivo construction of transgenes of the yellow–ASC locus. (B) Segments used for cloning are indicated by bars and labeled with 5′ yellow (blue), F, F–R, G, K, and J (orange). The size of each segment is indicated in kilobases. The w marker gene serves to identify transgenic flies. (C) yellow–ASC genomic region. The positions of each segment and primers for the molecular characterization of gap repair events are indicated. The y1 mutation is a point mutation in the yellow start codon; the mobile element constructs, however, contain a wild-type start codon. Primer numbers indicate the position relative to and downstream of the y transcription start. (D) DSB repair produced a mosaic phenotype. This fly carries both the F construct and the heat-shock-inducible I-SceI transgene. A heat shock has been applied at larval stages to induce DSBs. Dark patches (y+) are gap repair events (green arrows). The symbols in the boxed legend are also used in Figures 2 and 3.