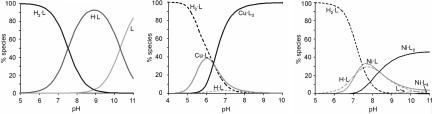
Fig. 3.
Speciation of L in aqueous solution as a function of pH in the absence of metal(II) (Left), in the presence of Cu(II) (Center), and in the presence of Ni(II) (Right). [L]total = 1 mM; [Cu(II)]total = 0.5 mM; and [Ni(II)]total = 0.33 mM. In the presence of metal(II), solid lines represent metal(II) complexes, and dashed lines represent species without metal(II). Charges are omitted for clarity.