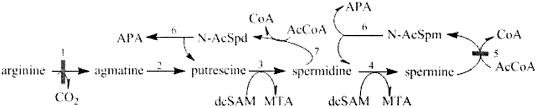
FIG. 1.
Pathway of polyamine metabolism by C. parvum. C. parvum differs from mammalian cells in several ways. The initial step of the forward pathway is catalyzed by arginine decarboxylase (step 1) instead of ornithine decarboxylase. The agmatine formed is converted into putrescine by agmatine iminohydrolase (step 2). In common with the mammalian pathway, aminopropyl groups are subsequently added to form spermidine and spermine by spermidine synthase (step 3) and spermine synthase (step 4), respectively. The reverse pathway in the parasite operates at a higher rate than the forward pathway and involves the acetylation of host-derived spermine by spermidine/spermine N1-acetyltransferase (step 5), and the N1-acetylspermine (N-AcSpm) formed is oxidized to spermidine and aminopropionaldehyde (APA) by polyamine oxidase (step 6). Spermidine can also be metabolized to putrescine and APA by the consecutive action of spermidine N1-acetyltransferase (N-AcSpd) (step 7) and a polyamine oxidase (step 6). In the mammalian cell, spermine can also be directly oxidized to spermidine by spermine oxidase, which is absent in C. parvum. Blocking C. parvum SSAT and ADC using DFMA and CGC-11157 (horizontal gray bar) will effectively compromise parasite polyamine metabolism with minimal effect upon the host, which lacks ADC and has a functional spermine oxidase, allowing the reverse polyamine pathway to function. dcSAM, decarboxylated S-adenosylmethionine; MTA, methylthioadenosine.