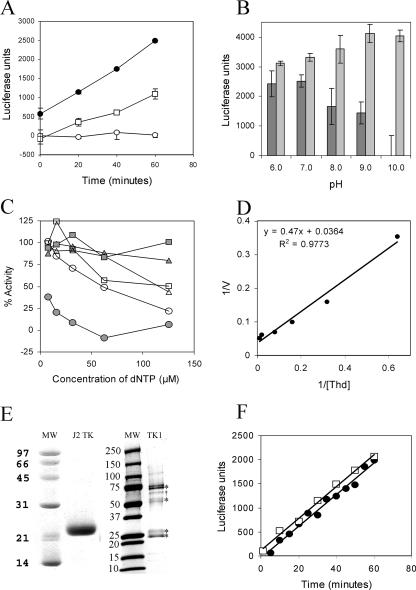
FIG. 2.
Characteristics of the enzymatic activity of J2 TK and human TK1. (A) ATP utilization by VV TK was dependent on either 100 μM thymidine (filled circles) or 100 μM IDU (open squares), while no ATP was consumed in the absence of a nucleoside substrate (open circles). (B) Thymidine-dependent utilization of ATP by VV TK was determined at pH 6, 7, 8, 9, and 10. Luciferase activity was measured following a kinase reaction in the presence (dark gray bars) and absence (light gray bars) of 50 μM thymidine. (C) VV TK enzymatic activity was determined in the presence of the potential allosteric effector molecules shown. Shaded circles and open circles represent dTTP and dUTP, respectively, while shaded squares represent the control without added inhibitors. Open triangles, shaded triangles, and open squares represent dGTP, dCTP, and dATP, respectively. (D) A Lineweaver-Burk plot for thymidine as a substrate of VV TK. (E) Coomassie-stained gels of purified J2 TK and human TK1. Higher-molecular-weight species indicated by asterisks in the human TK1 lane were identified as TK1 by mass spectrometry and are consistent with multimers. One contaminating bacterial protein was also identified as indicated by the dagger (†) symbol and is a bacterial protein of unknown function (b2255, gi16130190). (F) Enzymatic assays for both J2 (filled circles) and TK1 (open squares) are linear with time, and both require thymidine or a related substrate for ATP utilization.