Abstract
Candida dubliniensis isolates tested for susceptibility to anidulafungin, caspofungin, and micafungin commonly showed artifactual regrowth and/or trailing effects with MIC tests done under conditions involving a high initial yeast concentration. The artifacts were less common with Candida albicans and seldom seen for either species under Clinical and Laboratory Standards Institute method M27-A test conditions.
Candida albicans and Candida dubliniensis are two closely related yeast species associated with human commensalism and opportunistic infection (16). The echinocandin antifungal class is represented by three agents, anidulafungin (17), caspofungin (9), and micafungin (3). They are cyclic lipopeptides that inhibit β-1:3-d-glucan synthesis in fungal cell walls (11).
Susceptibility tests with echinocandins against yeasts can be done by using the Clinical and Laboratory Standards Institute (CLSI) M27-A method, read visually at 24 h (4), or by the European Committee on Antimicrobial Susceptibility Testing (EUCAST) method, which utilizes a 100-fold-higher initial yeast concentration, a higher (2%) glucose concentration, and spectrophotometric readings to determine 50% endpoints (5). A “paradoxical effect” has been reported for some C. albicans isolates, in which fungal growth recurs at caspofungin concentrations above the MIC (14, 15). Trailing yeast growth has been noted in tests of triazole antifungal agents against some yeast isolates but does not indicate resistance of clinical relevance (1, 13). We recently noticed both phenomena in our routine susceptibility tests with echinocandins and C. dubliniensis isolates and have therefore studied them further.
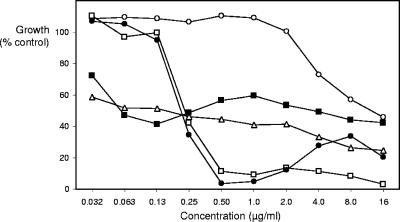
A panel of 20 C. dubliniensis isolates was selected at random, and a panel of 20 C. albicans isolates was chosen to include genome-sequenced strain SC5314, 10 randomly selected recent clinical isolates, Y109, a reduced-susceptibility clinical isolate (12), and 8 laboratory mutants of strain SC5314 with reduced echinocandin susceptibility (6, 7). Anidulafungin, caspofungin, and micafungin pure substances were donated, respectively, by Pfizer United Kingdom, Merck & Co., Inc., and Fujisawa GmBH, and were prepared as a series of aqueous doubling dilutions. Inocula for the M27-A and EUCAST tests were grown overnight at 30°C in neopeptone-yeast extract-glucose (NGY) medium (8) and added to morpholinepropanesulfonic acid (MOPS)-buffered RPMI 1640 medium (10) for M27-A tests or to the same medium with 2% glucose for EUCAST tests, with initial concentrations of 103 yeasts/ml and 105 yeasts/ml, respectively. Plates were incubated at 35°C for 24 h. M27-A test MICs were read visually as the lowest concentration at which a marked decrease in visible growth occurred (4), and paradoxical or trailing growth was noted. EUCAST method MICs were read from triplicate spectrophotometric dose-response data as 50% and 80% growth reduction endpoints. Trailing growth was defined as a difference greater than 100-fold between the two endpoints and paradoxical effects as an increase in growth of 5% or more above the dose-response minimum level. Figure 1 illustrates the application of these definitions.
FIG. 1.
Examples of dose-response curves for isolates tested showing normal echinocandin susceptibility and a typically sigmoid dose response (open squares) for caspofungin versus C. dubliniensis 75/043; reduced susceptibility and a typical dose-response curve (open circles) for caspofungin versus C. albicans T26; a trailing growth effect (open triangles) for anidulafungin versus C. dubliniensis B71507; a paradoxical regrowth effect (closed circles) for caspofungin versus C. dubliniensis 910Aa); and a trailing growth plus paradoxical effect (closed squares) for micafungin versus C. dubliniensis J931021.
MIC test results for the C. albicans and C. dubliniensis isolate panels are shown in Table 1. Anidulafungin and caspofungin MIC results for quality control (QC) strains ATCC 6258 and ATCC 22019 fell within published limits for the M27-A tests (4). For EUCAST tests, the caspofungin dose-response curve for ATCC 22019 showed a paradoxical effect, and the 50% endpoint result for ATCC 4258 was one dilution higher than that for the M27-A test QC range (no official EUCAST QC ranges have been published).
TABLE 1.
MICs of three echinocandin antifungal agents against Candida isolates by M27-A and EUCAST methods
| Species | Strain reference no. | 50% Inhibitory concn (μg/ml) at 24 ha
|
|||||
|---|---|---|---|---|---|---|---|
| M27-A test
|
EUCAST test
|
||||||
| Anidulafungin | Caspofungin | Micafungin | Anidulafungin | Caspofungin | Micafungin | ||
| C. albicans | SC5314 | ≤0.032 | 0.5 | ≤0.032 | ≤0.032 | 0.25 (P) | ≤0.032 |
| C. albicans | SCS140136P | ≤0.032 | 0.5 | ≤0.032 | ≤0.032 | 0.25 (P) | ≤0.032 |
| C. albicans | SCS67023C | ≤0.032 | 0.5 | ≤0.032 | ≤0.032 | 0.25 (P) | ≤0.032 |
| C. albicans | SCS68858 | ≤0.032 | 0.5 | ≤0.032 | ≤0.032 | 0.25 | ≤0.032 |
| C. albicans | AM2003/0083 | ≤0.032 | 0.5 | ≤0.032 | ≤0.032 | 0.25 | ≤0.032 |
| C. albicans | B06-341 | 0.13 | 0.13 | ≤0.032 | 0.13 | 0.5 | ≤0.032 |
| C. albicans | HK04M102095 | ≤0.032 | 0.5 | ≤0.032 | ≤0.032 | 0.25 | ≤0.032 |
| C. albicans | JIMS101103 | NG | NG | NG | ≤0.032 | 0.25 | ≤0.032 |
| C. albicans | JIMS132203 | ≤0.032 | 0.5 | ≤0.032 | ≤0.032 | 0.25 | ≤0.032 |
| C. albicans | JIMS146204 | ≤0.032 | 0.5 | ≤0.032 | ≤0.032 | 0.25 | ≤0.032 |
| C. albicans | NCPF6525 | 0.13 | 0.13 | ≤0.032 | 0.25 (P) | 1 (P) | ≤0.032 |
| C. albicans | T28 | ≤0.032 | 0.25 | ≤0.032 | ≤0.032 | 0.13 (P) | ≤0.032 |
| C. albicans | T32 | ≤0.032 | 0.25 | ≤0.032 | ≤0.032 | 0.13 (P) | ≤0.032 |
| C. albicans | NR2 | 0.5 | 4 | 0.25 | 0.25 | 4 (P) | 0.5 |
| C. albicans | NR3 | 1 | 8 | 1 | 1 | 4 (P) | 2 |
| C. albicans | NR4 | 0.25 | 2 | 0.25 | 0.13 | 2 (P) | 0.5 |
| C. albicans | CAI4RI | 0.5 | 4 | 0.25 | 0.25 | 4 (P) | 0.5 |
| C. albicans | T25 | 1.0 | 8 | 1.0 | 0.5 | 8 | 1 |
| C. albicans | T26 | 1.0 | 8 | 1.0 | 0.5 | 16 | 1 |
| C. albicans | Y109 | 0.25 | 4 | 0.25 | 0.13 | 2 | 0.25 |
| C. dubliniensis | 75/043 | ≤0.032 | 0.25 | ≤0.032 | ≤0.032 | 0.25 | ≤0.032 |
| C. dubliniensis | 81/060 | NG | NG | NG | ≤0.032 | 0.25 (P) | ≤0.032 |
| C. dubliniensis | 90/033 | ≤0.032 | NG | ≤0.032 | 8 | 16 | 0.063 (P, T) |
| C. dubliniensis | 910Aa | ≤0.032 | 0.5 | ≤0.032 | ≤0.032 (P) | 0.25 (P) | ≤0.032 (P, T) |
| C. dubliniensis | AMCC | ≤0.032 | 0.5 | ≤0.032 | ≤0.032 (T) | 0.25 (P) | ≤0.032 (T) |
| C. dubliniensis | ANSA 23a | NG | NG | NG | ≤0.032 | 0.25 (P) | ≤0.032 |
| C. dubliniensis | B71507 | ≤0.032 | 0.5 | ≤0.032 | 0.25 (T) | 8 | 0.063 (T) |
| C. dubliniensis | FS | ≤0.032 | 0.5 | ≤0.032 | 4 | 16 | 0.063 (P, T) |
| C. dubliniensis | IK | ≤0.032 | 0.5 | ≤0.032 | ≤0.032 (T) | 0.25 (P) | ≤0.032 (P, T) |
| C. dubliniensis | J930936 | ≤0.032 | 0.5 | ≤0.032 | ≤0.032 (T) | 0.25 | ≤0.032 (P, T) |
| C. dubliniensis | J931021 | ≤0.032 | 0.5 | ≤0.032 | 16 | 16 | 0.063 (P, T) |
| C. dubliniensis | KR | ≤0.032 | 0.5 | ≤0.032 | ≤0.032 (T) | 0.25 (P, T) | ≤0.032 (P, T) |
| C. dubliniensis | Leic875 | ≤0.032 | 0.5 | ≤0.032 | ≤0.032 (P) | 0.25 (P) | ≤0.032 (P, T) |
| C. dubliniensis | Leic952 | ≤0.032 | 0.5 | ≤0.032 | 8.0 | 0.25 (P, T) | 0.063 (P, T) |
| C. dubliniensis | SCS17154969-1 | ≤0.032 | 0.5 | ≤0.032 | ≤0.032 (P, T) | 0.5 (P) | 0.063 (P, T) |
| C. dubliniensis | SCS17154969-2 | ≤0.032 | 0.5 | ≤0.032 | 0.063 (T) | 0.5 (P) | 0.063 (T) |
| C. dubliniensis | SCS59662 | ≤0.032 | 0.5 | ≤0.032 | ≤0.032 (T) | 0.5 (P) | ≤0.032 (P, T) |
| C. dubliniensis | SCS65875C | ≤0.032 | 0.5 | ≤0.032 | ≤0.032 (T) | 8 | ≤0.032 (P, T) |
| C. dubliniensis | SCSBB417709 | ≤0.032 | 0.5 | ≤0.032 | ≤0.032 (T) | 0.25 (P) | ≤0.032 (T) |
| C. dubliniensis | ST | ≤0.032 | 0.5 | ≤0.032 | ≤0.032 (T) | 0.5 | 0.063 (T) |
| C. parapsilosis | ATCC 22019 | 0.5 | 1 | 0.25 | 0.5 | 1 (P) | 0.5 |
| C. krusei | ATCC 6258 | 0.13 | 1 | 0.13 | 0.063 | 2 | 0.13 |
NG, no growth; P, paradoxical effect; T, trailing growth.
For 24-h M27-A tests with C. albicans isolates, the control growth was too low for MIC readings with one isolate (Table 1). For 19 isolates, MICs of all three echinocandins indicated susceptibility to the agents, except for generally higher MICs with the clinical isolate Y109 and the laboratory-derived echinocandin-resistant mutants (6, 7). Similarly, among the 20 C. dubliniensis isolates tested by the M27-A method, 2 isolates gave insufficient control growth for determining a MIC, and the remaining 18 isolates were identically susceptible to all three echinocandins (Table 1). No examples of paradoxical growth or trailing growth were noted in these tests, as judged visually.
With EUCAST tests (Table 1), all 20 C. albicans isolates gave 24-h echinocandin MIC endpoints that were within one dilution of those determined by the M27-A test, except for caspofungin versus NCPF6525 and B06-341. A paradoxical effect was seen for one isolate tested with anidulafungin and for 10 of the 20 isolates tested with caspofungin. The EUCAST echinocandin 24-h test MICs for micafungin against the C. dubliniensis isolates were within one test dilution of those of M27-A test data. For four C. dubliniensis isolates, anidulafungin and caspofungin MICs were considerably higher with the EUCAST method than with the M27-A method. Paradoxical effects or trailing growth or both phenomena were commonly encountered with all three echinocandins (Table 1).
Since these results suggested that the paradoxical effects might be related to the larger inoculum size or higher glucose concentration in EUCAST tests, five C. dubliniensis isolates were retested for caspofungin susceptibility, using RPMI 1640 medium containing 0.2% and 2% glucose and initial concentrations of 103 yeasts/ml (M27-A), 104 yeasts/ml, and 105 yeasts/ml (EUCAST). Read both visually and by spectrophotometer, MIC results were similar under all glucose concentration and inoculum conditions for a 50% growth inhibition endpoint regardless of paradoxical or trailing effects. Neither effect was seen for tests at either glucose concentration at an initial concentration of 103 yeasts/ml. At 104 yeasts/ml, paradoxical regrowth occurred with 4/5 isolates at both glucose concentrations. At 105 yeasts/ml, paradoxical regrowth was again noted for 4/5 isolates in tests with 0.2% glucose and in 3/5 isolates in tests with 2% glucose.
Despite their close taxonomic similarities, C. albicans and C. dubliniensis differed markedly in their echinocandin responses under EUCAST MIC test conditions in vitro, probably because of the EUCAST test's higher initial yeast concentration. Inoculum size is known to influence the outcome of caspofungin susceptibility tests with C. albicans (2, 12). C. dubliniensis commonly showed trailing growth and/or paradoxical regrowth at high concentrations of all three echinocandins, whereas for C. albicans isolates, only paradoxical effects were seen. Under M27-A test conditions, the MIC endpoints for each echinocandin were more consistent and trailing growth and paradoxical regrowth were not encountered. However, 3 of the 42 isolates tested grew insufficiently under these conditions to allow MIC determination. Both methods offer 24-h echinocandin MICs. From our data, the M27-A test has the advantage of minimizing paradoxical and trailing growth effects with C. dubliniensis, while the larger inoculum size and higher glucose concentration with the EUCAST approach ensure adequate levels of control growth and generate portable data from spectrophotometric dose-response curves.
With both methodologies, 50% endpoints read at 24 h were similar for the majority of isolates of both yeast species if the paradoxical and trailing growth effects were ignored, suggesting that the effects are artifactual and unlikely to be of clinical relevance.
Acknowledgments
This study was supported by a grant from the European Union (EURESFUN).
Footnotes
Published ahead of print on 16 February 2007.
REFERENCES
- 1.Arthington-Skaggs, B. A., W. Lee-Yang, M. A. Ciblak, J. P. Frade, M. E. Brandt, R. A. Hajjeh, L. H. Harrison, A. N. Sofair, and D. W. Warnock for the Candidemia Active Surveillance Group. 2002. Comparison of visual and spectrophotometric methods of broth microdilution MIC end point determination and evaluation of a sterol quantitation method for in vitro susceptibility testing of fluconazole and itraconazole against trailing and nontrailing Candida isolates. Antimicrob. Agents Chemother. 462477-2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartizal, C., and F. C. Odds. 2003. Influences of methodological variables on susceptibility testing of caspofungin against Candida species and Aspergillus fumigatus. Antimicrob. Agents Chemother. 472100-2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chandrasekar, P. H., and J. D. Sobel. 2006. Micafungin: a new echinocandin. Clin. Infect. Dis. 421171-1178. [DOI] [PubMed] [Google Scholar]
- 4.CLSI. 2006. Quality control minimal inhibitory concentration (MIC) limits for broth microdilution and MIC interpretive breakpoints; informational supplement, 2nd ed. Clinical and Laboratory Standards Institute, Wayne, PA.
- 5.Cuenca-Estrella, M., A. Gomez-Lopez, E. Mellado, and J. L. Rodriguez-Tudela. 2005. Correlation between the procedure for antifungal susceptibility testing for Candida spp. of the European Committee on Antibiotic Susceptibility Testing (EUCAST) and four commercial techniques. Clin. Microbiol. Infect. 11486-492. [DOI] [PubMed] [Google Scholar]
- 6.Douglas, C. M., J. A. Dippolito, G. J. Shei, M. Meinz, J. Onishi, J. A. Marrinan, W. Li, G. K. Abruzzo, A. Flattery, K. Bartizal, A. Mitchell, and M. B. Kurtz. 1997. Identification of the FKS1 gene of Candida albicans as the essential target of 1,3-β-d-glucan synthase inhibitors. Antimicrob. Agents Chemother. 412471-2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kurtz, M. B., G. Abruzzo, A. Flattery, K. Bartizal, J. A. Marrinan, W. Li, J. Milligan, K. Nollstadt, and C. M. Douglas. 1996. Characterization of echinocandin-resistant mutants of Candida albicans: genetic, biochemical, and virulence studies. Infect. Immun. 643244-3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.MacCallum, D. M., H. Findon, C. C. Kenny, G. Butler, K. Haynes, and F. C. Odds. 2006. Different consequences of ACE2 and SWI5 gene disruptions for virulence of pathogenic and nonpathogenic yeasts. Infect. Immun. 745244-5248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCormack, P. L., and C. M. Perry. 2005. Caspofungin: a review of its use in the treatment of fungal infections. Drugs 652049-2068. [DOI] [PubMed] [Google Scholar]
- 10.NCCLS. 2002. Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard, 2nd ed., vol. 17, p. 9. National Committee for Clinical Laboratory Standards, Wayne, PA. [Google Scholar]
- 11.Odds, F. C., A. J. P. Brown, and N. A. R. Gow. 2003. Antifungal agents: mechanisms of action. Trends Microbiol. 11272-279. [DOI] [PubMed] [Google Scholar]
- 12.Odds, F. C., M. Motyl, R. Andrade, J. Bille, E. Cantón, M. Cuenca-Estrella, A. Davidson, C. Durussel, D. Ellis, E. Foraker, A. W. Fothergill, M. A. Ghannoum, R. A. Giacobbe, M. Gobernado, R. Handke, M. Laverdière, W. Lee-Yang, W. G. Merz, L. Ostrosky-Zeichner, J. Pemán, S. Perea, J. R. Perfect, M. A. Pfaller, L. Proia, J. H. Rex, M. G. Rinaldi, J. L. Rodriguez-Tudela, W. A. Schell, C. Shields, D. A. Sutton, P. E. Verweij, and D. W. Warnock. 2004. Interlaboratory comparison of results of susceptibility testing with caspofungin against Candida and Aspergillus species. J. Clin. Microbiol. 423475-3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rex, J. H., P. W. Nelson, V. L. Paetznick, M. Lozanochiu, A. Espinelingroff, and E. J. Anaissie. 1998. Optimizing the correlation between results of testing in vitro and therapeutic outcome in vivo for fluconazole by testing critical isolates in a murine model of invasive candidiasis. Antimicrob. Agents Chemother. 42129-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stevens, D. A., M. Espiritu, and R. Parmar. 2004. Paradoxical effect of caspofungin: reduced activity against Candida albicans at high drug concentrations. Antimicrob. Agents Chemother. 483407-3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stevens, D. A., T. C. White, D. S. Perlin, and C. P. Selitrennikoff. 2005. Studies of the paradoxical effect of caspofungin at high drug concentrations. Diagn. Microbiol. Infect. Dis. 51173-178. [DOI] [PubMed] [Google Scholar]
- 16.Sullivan, D. J., G. P. Moran, and D. C. Coleman. 2005. Candida dubliniensis: ten years on. FEMS Microbiol. Lett. 2539-17. [DOI] [PubMed] [Google Scholar]
- 17.Vazquez, J. A., and J. D. Sobel. 2006. Anidulafungin: a novel echinocandin. Clin. Infect. Dis. 43215-222. [DOI] [PubMed] [Google Scholar]