Abstract
In this study, we developed a new mariner-based transposition system for Listeria monocytogenes. The mariner-based system has a high rate of transposition and a low rate of plasmid retention, and transposition is very random, making it an ideal tool for high-throughput transposon mutagenesis in L. monocytogenes.
Listeria monocytogenes is a saprophytic gram-positive bacterial rod that is ubiquitous in nature and is an opportunistic food-borne pathogen of humans and a variety of other vertebrates (24). During infection, L. monocytogenes multiplies intracellularly in the cytosols of host cells (19, 23). Intracellular survival of L. monocytogenes relies largely on its ability to subvert host functions by escaping phagocytic vacuoles and spreading from cell to cell without exiting the intracellular milieu. In the last two decades, multiple studies have aimed at identifying virulence factors and deciphering the mechanisms by which L. monocytogenes survives in a wide range of environments (9, 13).
One of the most valuable genetic tools used to study bacteria is the transposon. Transposons can be used to perform high-throughput mutagenesis of an entire chromosome, generating banks of mutants that can be screened for identification of factors related to specific bacterial functions. The transposon delivery systems that are currently available for use with L. monocytogenes are not ideal for these types of studies (4, 7, 14). For example, the most commonly used transposon delivery system, Tn917-LTV3, is more than 22 kb in size and has a low efficiency of transposition and a high rate of vector retention (4). In this study, we aimed at designing a transposon delivery system that is more suitable to high-throughput mutagenesis.
In recent years, Himar1 mariner has been used as the transposon of choice in performing high-throughput mutagenesis in many different bacterial species, including low-GC-content gram-positive species (1, 2, 18). Himar1 was originally isolated from the horn fly, Haematobia irritans, and is a member of the Tc1/mariner superfamily of transposable elements (21). The Tc1/mariners are the most-widespread transposons in nature. These elements require no factors for transposition other than their self-encoded transposases (16), a feature that makes them ideal candidates for development into generalized genetic tools. Moreover, the mariner requirement for insertion is the dinucleotide TA, which makes it perfect for transposition into low-GC-content organisms such as L. monocytogenes (39% GC). We reasoned that a mariner-based transposition system would be an excellent tool for the entire community of scientists working with L. monocytogenes.
Construction of mariner-based transposon delivery vectors.
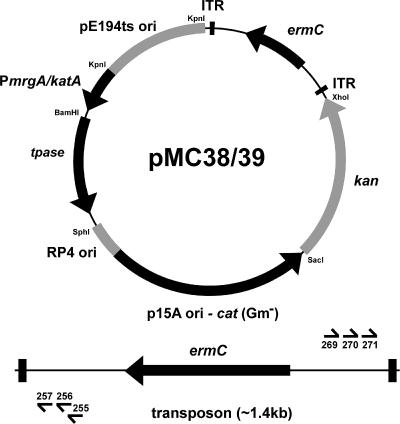
The plasmids and primers used for construction of a mariner delivery vector for L. monocytogenes are listed in Tables 1 and 2, respectively. pPL2 (17) and pDG780 (12) were digested with SacI and XhoI, and the gram-positive kanamycin (kan) resistance cassette from pDG780 was ligated into pPL2 to create pMC14. The gram-positive chloramphenicol acetyltransferase gene (cat) from pPL2 was amplified by PCR with primer pair Marq155/156 and ligated into pCR2.1-Topo (Invitrogen) to create pMC1. pMC1 was digested with BamHI and ligated into the BglII site of pMMOrf (15) between the 5′ and 3′ Himar1-inverted terminal repeats (ITR), creating pMC3. The Himar1 transposase gene (tpase) (16) was amplified by PCR from pNF1100 (provided by Nancy Freitag) with primer pair Marq188/234. The promoter regions of the Bacillus subtilis CU1065 (provided by John Helmann) mrgA and katA genes were amplified by PCR, using primer pairs Marq247/248 and Marq249/250, respectively. PmrgA, PkatA, and tpase PCR fragments were digested with BamHI, and each promoter was individually ligated to tpase. The PmrgA-tpase and PkatA-tpase ligation products were amplified by PCR with primer pairs Marq247/188 and Marq249/188, respectively. The PmrgA-tpase and PkatA-tpase PCR products and pMC14 were digested with KpnI and SphI. The pMC14 fragment comprising P4oriT, p15Aori, gram-negative cat, and gram-positive kan genes was ligated with PmrgA-tpase and PkatA-tpase, respectively. The ITR-cat-ITR fragment from pMC3 was ligated at the KpnI and XhoI sites, and the gram-positive cat gene was later replaced by ermC. This replacement was done by amplifying ermC from pPL3e (10) with primer pair Marq205/206, digesting the PCR products and vector with NdeI, and replacing the cat gene between the ITR with ermC. Last, the temperature-sensitive origin of replication pE194ts ori in pKSV7 (22) was amplified by PCR, using primer pair Marq194/195, digested with KpnI, and ligated into each vector, creating pMC38 (PmgrA) and pMC39 (PkatA) (Fig. 1). The vectors are 8,172 bp (pMC38) and 8,297 bp (pMC39), and the transposon itself is 1,395 bp.
TABLE 1.
Bacterial strains and vectors used in this study
| Strain or vector | Genotype or relevant feature | Reference or source |
|---|---|---|
| 10403S | L. monocytogenes serotype 1/2a | 3 |
| CU1065 | B. subtilis W168 trpC2 attSPβ | J. D. Helmann |
| DH5α | E. coli supE44 ΔlacU169 (φ80lacZΔM15) hsdR17 recA1 endA1 gyrA96 thi-1 relA1 | Lab stock |
| pCR2.1-TOPO | Cloning vector from Invitrogen | |
| pDG780 | Contains a gram-positive kan resistance cassette | 12 |
| pKSV7 | Shuttle vector with gram-positive pE194ts ori | 22 |
| pLTV3 | Tn917 delivery vector | 4 |
| pMC1 | Gram-positive cat gene from pPL2 cloned into pCR2.1-TOPO | This study |
| pMC3 | Gram-positive cat gene from pMC1 cloned into pMMOrf | This study |
| pMC14 | Gram-positive kan gene from pDG780 cloned into pPL2 | This study |
| pMC25 | mariner delivery vector with L. monocytogenes promoter PP60 | This study |
| pMC30 | mariner delivery vector with L. monocytogenes promoter PactA | This study |
| pMC38 | mariner delivery vector with B. subtilis promoter PmrgA | This study |
| pMC39 | mariner delivery vector with B. subtilis promoter PkatA | This study |
| pMMOrf | Contains 5′ and 3′ ITR from Himar1 | 15 |
| pNF1100 | Contains a copy of Himar1 derived from pMEnt-neo | N. E. Freitag; 16 |
| pPL2 | Site-specific shuttle integration vector | 17 |
| pPL3e | pPL2 derivative with gram-positive ermC gene | 10 |
TABLE 2.
Oligonucleotide primers used in this study
| No. of primer | Sequence 5′→3′a | Characteristic (reference) |
|---|---|---|
| 155 | TTGGATCCCGGAGACGGTCACA | BamHI |
| 156 | CGCATCTGTGCGGTATTTCA | |
| 188 | ATCCGCATGCTGCAAGGCGATTAAGT | SphI |
| 194 | CGGGTACCATCACACGCAAAAAGGA | KpnI |
| 195 | CGGGTACCTAAATTCAAAATCTATC | KpnI |
| 205 | GGTATAGCATATGAATCGCATCCGATTGCAG | NdeI |
| 206 | TGTCAGACATATGGGCACACGAAAAACAAGT | NdeI |
| 207 | GGCCACGCGTCGACTAGTACNNNNNNNNNNGTAAT | ARB1B (8) |
| 208 | GGCCACGCGTCGACTAGTAC | ARB2 (8) |
| 234 | GCGGATCCAGAGGAGTTTTATGAATATGGAAAAAAAGGAATTTCGTGTTT | BamHI and RBS |
| 247 | GCGGTACCTATCATCAATACTATA | KpnI |
| 248 | CAGGATCCGTGATCTGTTGACTTAAT | BamHI |
| 249 | GCGGTACCTTTTCTTTGATGCTGA | KpnI |
| 250 | GCGGATCCAATTTATAAGAACATAAT | BamHI |
| 254 | CGTGGAATACGGGTTTGCTAAAAG | |
| 255 | CAGTACAATCTGCTCTGATGCCGCATAGTT | |
| 256 | TAGTTAAGCCAGCCCCGACACCCGCCAACA | |
| 257 | CTTACAGACAAGCTGTGACCGTCT | |
| 269 | GCTCTGATAAATATGAACATGATGAGTGAT | |
| 270 | TGTGAAATACCGCACAGATGCGAAGGGCGA | |
| 271 | GGGAATCATTTGAAGGTTGGTACT |
Restriction sites are underlined, and the ribosome binding site is italicized.
FIG. 1.
Physical map of the mariner-based transposon delivery vectors pMC38 and pMC39. The vectors comprise the Escherichia coli p15A low-copy-number replication origin (5), the RP4 ori for conjugative transfer (20), the pE194ts gram-positive temperature-sensitive replication origin (11, 25), a gram-negative chloramphenicol resistance gene (cat) (5), a gram-positive noninducible erythromycin resistance gene (ermC) (25) flanked by the 29-bp ITR of the Himar1 mariner (15), a gram-positive kanamycin resistance gene (kan) (12) as a screening marker for loss of the plasmid, and the Himar1 mariner transposase gene (tpase) (16). The indicated restriction sites represent those used for cloning, but they are not necessarily unique. Gm−, gram negative.
The Himar1 transposase and the ITR sequences are the only two factors required for transposition; thus, successful expression of the transposase in L. monocytogenes is the key element. In our initial constructs, the L. monocytogenes p60 (pMC25) and actA (pMC30) promoters were used to direct transcription of the transposase gene. This approach failed, as the entire plasmid invariably integrated into the chromosome. However, when we used B. subtilis to evaluate the efficiency of transposition with these same vectors, we obtained a very low level of plasmid retention (data not shown). We reasoned that the Listeria species promoters were responsible for mediating plasmid integration into the chromosome of L. monocytogenes and sought to use two B. subtilis σA-dependent promoters (PmrgA and PkatA) with no sequence similarity to the Listeria genome (6). This approach was very successful.
Evaluation of the mariner-based transposon delivery vector.
pMC38 and pMC39 were preferentially transferred into L. monocytogenes strain 10403S by electroporation, as the efficacy of transfer by conjugation was very low. Transformants were selected at 30°C on brain heart infusion (BHI) plates supplemented with erythromycin at 5 μg/ml. Individual colonies were grown overnight in BHI with erythromycin and kanamycin (10 μg/ml) at 30°C with shaking. The cultures were diluted 1/200 in broth with erythromycin, grown for 1 h at 30°C with shaking, and then shifted to 40°C for about 6 h until the optical density at 600 nm was between 0.3 and 0.5. Aliquots of the culture were plated on BHI agar supplemented with erythromycin and incubated at 40°C. Individual colonies were picked and plated in parallel on BHI agar supplemented with either erythromycin or kanamycin to evaluate the rate of plasmid retention. The rate of plasmid retention was calculated by dividing the number of kanamycin-resistant colonies (due to plasmid retention) by the number of erythromycin-resistant colonies (total number of mutants with the plasmid or with the transposon only). The same procedures were used to evaluate the Tn917-based transposition system, Tn917-LTV3 (4), except that 1 μg/ml erythromycin plus 25 μg/ml lincomycin were used for selection of transposon mutants and 12.5 μg/ml tetracycline was used to evaluate plasmid retention.
In general, we obtained 10-fold-more mutants with the mariner-based vectors than with the Tn917-based vector (Table 3). Plasmid retention was less than 2.5% for the mariner-based vectors, whereas it was more than 50% with the Tn917-based vector. Therefore, by comparing the numbers of transposon insertion mutants (colonies that have lost the plasmid) generated by the two systems, we estimated that the efficiency of transposition of the mariner-based vectors was more than 20-fold higher than that of the Tn917-based vector.
TABLE 3.
Comparison of transposon delivery vectors
| Delivery vector | Size of vector | Size of transposon (bp) | Total no. of mutants (CFU/ml ± SD)a | % ± SD of CFUs bearing vector |
|---|---|---|---|---|
| pLTV3(4) | 22.1 kb | 14,861 | (9.80 ± 2.00) × 104 | >50b |
| pMC38 | 8,172 bp | 1,395 | (1.73 ± 1.07) × 106 | 2.4 ± 1.7 |
| pMC39 | 8,291 bp | 1,395 | (1.08 ± 0.76) × 106 | 0.85 ± 0.01 |
The total number of mutants generated by each vector was obtained from the results of three independent libraries for pLTV3 and pMC38 and two independent libraries for pMC39. SD, standard deviation.
Estimated number.
To evaluate the randomness of transposition, we arbitrarily picked 100 erythromycin-resistant colonies from one library, performed Southern blot analysis, and identified the sites of transposon insertion. For Southern blot analysis, a 400-bp fragment within the ermC gene was amplified from pMC38 by PCR, using primer pair Marq206/254. The probe was labeled with alkaline phosphatase, using the AlkaPhos Direct labeling system from Amersham Biosciences. Of the 100 mutants, 84 had a single transposon insertion, whereas 16 had two-to-three insertions (data not shown). To identify the sites of transposon insertion, we initially performed arbitrary PCR to amplify the DNA sequences flanking the transposon (8). DNA was amplified from either end of the transposon with a series of two rounds of PCR with Taq polymerase in the first round and Expand High Fidelity polymerase (Roche) in the second round. In each round, a transposon-specific primer and an arbitrary primer were used. The arbitrary primers Marq207 and Marq208 were previously identified as ARB1B and ARB2 by Garsin et al. (8). The approximate locations of transposon-specific primers are illustrated in Fig. 1. In the first round, DNA fragments from the left and right ends of the transposon were amplified with primer pairs Marq207/255 and Marq207/269, respectively. For the second round, 5 μl of a 1/25 dilution from the first round of PCR was used in a 20-μl reaction. DNA fragments from the left and right ends of the transposon were amplified with primer pairs Marq208/256 and Marq208/270, respectively. The PCR products were sequenced, using primers Marq257 and Marq271 for the left and right ends of the transposon, respectively. The Biotechnology Research Center of Cornell University performed the sequencing with an Applied Biosystems automated 3730 DNA analyzer. The ListiList website (http://genolist.pasteur.fr/ListiList/) was used for sequence analysis.
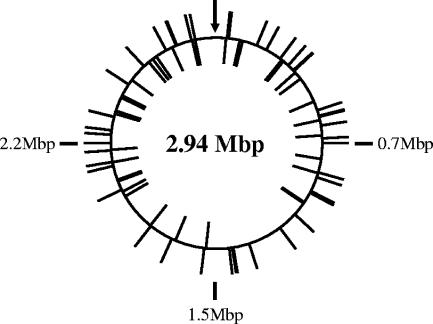
Readable sequencing results were obtained from 77 transposon insertion mutants, including one pair of siblings and a series of three genes that were hit twice. For the 77 mutants, 36 insertions were in positive-strand open reading frames, 29 in negative-strand open reading frames, and 12 in intergenic regions (Fig. 2). The mutants distributed evenly along the L. monocytogenes genome, and there was no bias in terms of the transposon orientation. We further aligned all the insertion sites, and no sequence specificity beyond the known requirement of the dinucleotide TA was found (data not shown).
FIG. 2.
Locations of 77 sequenced Himar1 insertions in the chromosome of L. monocytogenes. The bars located outside the circle indicate transposons that were oriented in the same direction as the positive strand, whereas the bars located inside the circle indicate transposons that were oriented in the opposite direction. Each quarter of the chromosome is identified with the approximate base pair, except for bp 1, which is identified by an arrow.
Taken together, these results show that the newly designed mariner delivery vectors are powerful genetic tools to study L. monocytogenes. The mariner-based system outachieves the Tn917-based system in the following aspects: transposition efficiency, randomness, and a low rate of plasmid retention. The temperature-sensitive replicon (pE194ts ori) and the noninducible erythromycin resistance gene (ermC) are common to many gram-positive bacteria. We believe that this mariner-based system will be a great tool for the entire community of scientists working with L. monocytogenes and other low-GC gram-positive bacteria.
Acknowledgments
We thank Nancy Freitag for providing the plasmid pNF1100, John Helmann for providing the Bacillus subtilis CU1065 strain and the plasmid pDG780, Darren Higgins for providing the plasmid pPL3e, David Lampe for providing the plasmid pMMORF, and Daniel Portnoy for providing the plasmid pPL2. We are grateful to Joe Peters for helpful suggestions and discussions and to Emily Slepkov for careful reading of the manuscript.
This work was in part supported by U.S. Public Health Service grant AI52154 to H.M. from NIAID.
Footnotes
Published ahead of print on 16 February 2007.
REFERENCES
- 1.Akerley, B. J., E. J. Rubin, A. Camilli, D. J. Lampe, H. M. Robertson, and J. J. Mekalanos. 1998. Systematic identification of essential genes by in vitro mariner mutagenesis. Proc. Natl. Acad. Sci. USA 95:8927-8932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bae, T., A. K. Banger, A. Wallace, E. M. Glass, F. Aslund, O. Schneewind, and D. M. Missiakas. 2004. Staphylococcus aureus virulence genes identified by bursa aurealis mutagenesis and nematode killing. Proc. Natl. Acad. Sci. USA 101:12312-12317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bishop, D. K., and D. J. Hinrichs. 1987. Adoptive transfer of immunity to Listeria monocytogenes: the influence of in vitro stimulation on lymphocyte subset requirements. J. Immunol. 139:2005-2009. [PubMed] [Google Scholar]
- 4.Camilli, A., D. A. Portnoy, and P. Youngman. 1990. Insertional mutagenesis of Listeria monocytogenes with a novel Tn917 derivative that allows direct cloning of DNA flanking transposon insertions. J. Bacteriol. 172:3738-3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang, A. C., and S. N. Cohen. 1978. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J. Bacteriol. 134:1141-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen, L., L. Keramati, and J. D. Helmann. 1995. Coordinate regulation of Bacillus subtilis peroxide stress genes by hydrogen peroxide and metal ions. Proc. Natl. Acad. Sci. USA 92:8190-8194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaillard, J. L., P. Berche, and P. Sansonetti. 1986. Transposon mutagenesis as a tool to study the role of hemolysin in the virulence of Listeria monocytogenes. Infect. Immun. 52:50-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garsin, D. A., J. Urbach, J. C. Huguet-Tapia, J. E. Peters, and F. M. Ausubel. 2004. Construction of an Enterococcus faecalis Tn917-mediated-gene-disruption library offers insight into Tn917 insertion patterns. J. Bacteriol. 186:7280-7289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gray, M. J., N. E. Freitag, and K. J. Boor. 2006. How the bacterial pathogen Listeria monocytogenes mediates the switch from environmental Dr. Jekyll to pathogenic Mr. Hyde. Infect. Immun. 74:2505-2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gründling, A., L. S. Burrack, H. G. Bouwer, and D. E. Higgins. 2004. Listeria monocytogenes regulates flagellar motility gene expression through MogR, a transcriptional repressor required for virulence. Proc. Natl. Acad. Sci. USA 101:12318-12323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gryczan, T. J., J. Hahn, S. Contente, and D. Dubnau. 1982. Replication and incompatibility properties of plasmid pE194 in Bacillus subtilis. J. Bacteriol. 152:722-735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guérout-Fleury, A. M., K. Shazand, N. Frandsen, and P. Stragier. 1995. Antibiotic-resistance cassettes for Bacillus subtilis. Gene 167:335-336. [DOI] [PubMed] [Google Scholar]
- 13.Hamon, M., H. Bierne, and P. Cossart. 2006. Listeria monocytogenes: a multifaceted model. Nat. Rev. Microbiol. 4:423-434. [DOI] [PubMed] [Google Scholar]
- 14.Kathariou, S., P. Metz, H. Hof, and W. Goebel. 1987. Tn916-induced mutations in the hemolysin determinant affecting virulence of Listeria monocytogenes. J. Bacteriol. 169:1291-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lampe, D. J., B. J. Akerley, E. J. Rubin, J. J. Mekalanos, and H. M. Robertson. 1999. Hyperactive transposase mutants of the Himar1 mariner transposon. Proc. Natl. Acad. Sci. USA 96:11428-11433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lampe, D. J., M. E. Churchill, and H. M. Robertson. 1996. A purified mariner transposase is sufficient to mediate transposition in vitro. EMBO J. 15:5470-5479. [PMC free article] [PubMed] [Google Scholar]
- 17.Lauer, P., M. Y. N. Chow, M. J. Loessner, D. A. Portnoy, and R. Calendar. 2002. Construction, characterization, and use of two Listeria monocytogenes site-specific phage integration vectors. J. Bacteriol. 184:4177-4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Le Breton, Y., N. P. Mohapatra, and W. G. Haldenwang. 2006. In vivo random mutagenesis of Bacillus subtilis by use of TnYLB-1, a mariner-based transposon. Appl. Environ. Microbiol. 72:327-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mounier, J., A. Ryter, M. Coquis-Rondon, and P. J. Sansonetti. 1990. Intracellular and cell-to-cell spread of Listeria monocytogenes involves interaction with F-actin in the enterocytelike cell line Caco-2. Infect. Immun. 58:1048-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pansegrau, W., G. Ziegelin, and E. Lanka. 1988. The origin of conjugative IncP plasmid transfer: interaction with plasmid-encoded products and the nucleotide sequence at the relaxation site. Biochim. Biophys. Acta 951:365-374. [DOI] [PubMed] [Google Scholar]
- 21.Plasterk, R. H., Z. Izsvak, and Z. Ivics. 1999. Resident aliens: the Tc1/mariner superfamily of transposable elements. Trends Genet. 15:326-332. [DOI] [PubMed] [Google Scholar]
- 22.Smith, K., and P. Youngman. 1992. Use of a new integrational vector to investigate compartment-specific expression of the Bacillus subtilis spoIIM gene. Biochimie 74:705-711. [DOI] [PubMed] [Google Scholar]
- 23.Tilney, L. G., and D. A. Portnoy. 1989. Actin filaments and the growth, movement, and spread of the intracellular bacterial parasite, Listeria monocytogenes. J. Cell Biol. 109:1597-1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vázquez-Boland, J. A., M. Kuhn, P. Berche, T. Chakraborty, G. Dominguez-Bernal, W. Goebel, B. González-Zorn, J. Wehland, and J. Kreft. 2001. Listeria pathogenesis and molecular virulence determinants. Clin. Microbiol. Rev. 14:584-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Villafane, R., D. H. Bechhofer, C. S. Narayanan, and D. Dubnau. 1987. Replication control genes of plasmid pE194. J. Bacteriol. 169:4822-4829. [DOI] [PMC free article] [PubMed] [Google Scholar]